Part 6: THERMODYNAMICS
This should have been a reasonably straight forward calculation, but care needs to be taken with the last part of the question.
a. Cyclohexane and trans-hex-3-ene are constitutional isomers. (1 mark)
b. C6H12 + 9 O2 --> 6 CO2 + 6 H2O (1.5 marks)
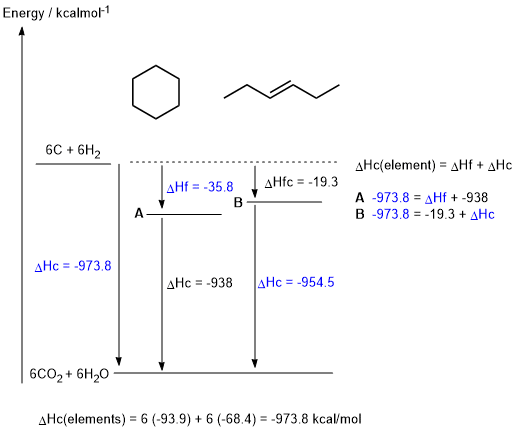
c. (see figure)
DHc elements = (6 x -93.9) + (6 x -68.4) = -973.8 kcal/mol
DHf A= -973.8 - (- 938) = -35.8 kcal/mol
DHc B = -973.8 - (- 19.3) = -954.5 kcal/mol
(1.5 marks)
d.

e.
The structures are shown in the figure above (0.5 marks each)
Given the thermodynamic data in the table provided in the question, and hence the figure shown above, A is the most stable isomer (i.e. lowest energy) (1 mark)
Several factors to consider here, first the more stable isomer will be composed of more stronger covalent bonds.
The acyclic alkene has a C=C and hence 2 x sp2 C and 2 primary sp3 C atoms while the cycloalkane has 6 secondary sp3 C atoms and therefore 12 secondary sp3 C-H bonds.
The pi bond in the alkene is about 20 kcal/mol weaker than the extra C-C sigma bond in cyclohexane.
Primary C-H bonds are about 3 kcal/mol stronger than secondary C-H bonds.
Allylic C-H bonds (there are 4 in the alkene) are about 10 kcal/mol weaker than equivalent simple C-H bonds.
sp2 C-H bonds (there are 2 in the alkene) are about 10 kcal/mol stronger than sp3 C-H bonds.
All told, this approximations suggests that cyclohexane is about 20 kcal/mol more stable than the alkene
(1.5 marks)
Common errors: