Part 7: MECHANISMS
Note that no other reagents are needed in order to complete any
of these sequences, you should only be using what is there.
A i

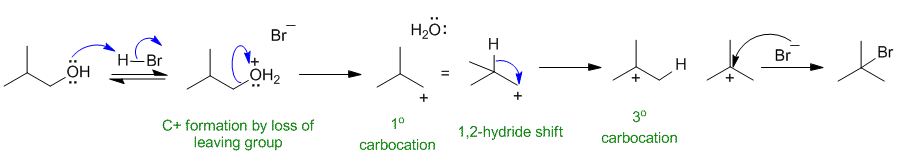
Since the product has the FG position changed, there has been a rearrangement of a C+, hence we need to show an Sn1 mechanism by protonating the -OH to make a better leaving group.
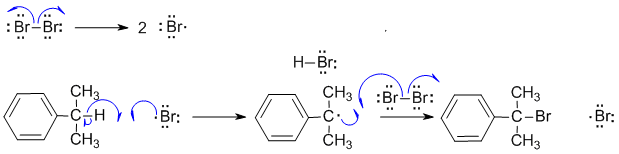
A ii Radical halogenation reactions. First step is the formation of the halogen radical by dissociation of the halogen. Note that the Br-Br bond is weak and readily broken when heated. The Br radical then abstracts the tertiary benzylic H to give the resonance stablised tertiary radical and this in turn leads to the tertiary bromide. Note that a bromine radical is also produced and this means the process can continue (it's a cyclic process). Note that because we are talking about radicals, we need to use fishhook type curly arrows.
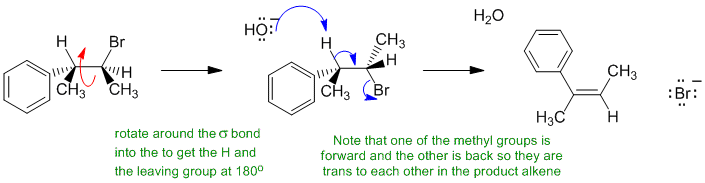
B i If an alkyl bromide is heated with strong base (here it's ethoxide), then a 1,2-elimination occurs to give an alkene. Alkyl halide eliminations are typically E2. This prefers an anti arrangement (180 degrees) of the C-H and C-LG bonds. In this reaction, the base is hydroxide (i.e. small), so the Zaistev elimination is going to dominate to give the more highly substituted alkene and the major product will be the more stable alkene (conjugated system) where the methyl groups are trans.
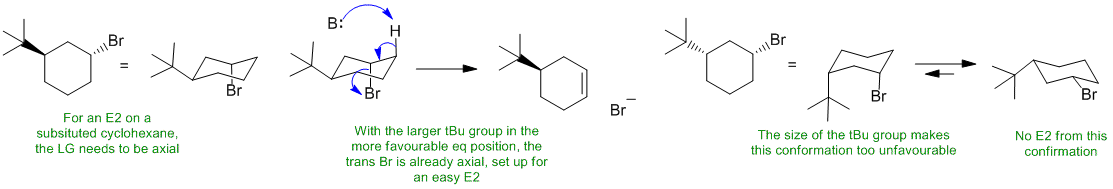
B ii An alkyl bromide will react with a strong (bulky) base and heat via an E2 pathway. In a cyclohexyl system, the Br will need to be axial to get the required anti (180 degree) alignment with the H-C and C-Br bonds. However, the t-butyl substituent has a very strong preference to be in the more favourable equatorial position. In the trans isomer (A) with the tBu equatorial, the Br is already axial so the E2 reaction will be fast. In the cis isomer (B), the Br can't get to the axial position because the size of the tBu stops the required ring flip... no E2 (maybe a much slower E1).
Common errors:
General:
i. Drawing curly arrows that were backwards... always electron rich to electron poor. ALWAYS! Or worse, not drawing arrows for some steps.
ii. Not balancing charges in each mechanistic step.
iii. Not providing justifications / curly arrows for the questions that asked for them.
iv. Compressing several reactions steps in to one step and therefore omitting / ignoring important intermediates.