Part
6: THERMODYNAMICS
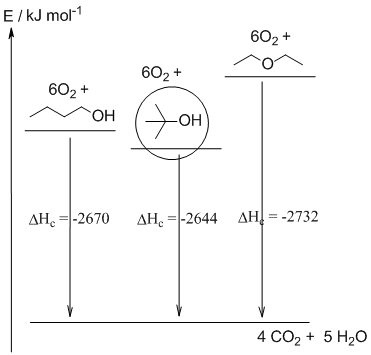
a. C4H10O + 6O2 -----> 4 CO2 + 5 H2O
b.

c. tert-butanol is more stable than n-butanol. This is because tert-butanol, the more branched isomer has more primary C-H bonds (9 in tert-butanol compared to just 3 in 1-butanol) and primary C-H bonds are stronger than secondary C-H bonds (which in trun are stronger than tertiary C-H bonds). More stronger bonds (intramolecular forces) means a more stable isomer.
d. Boiling point is a physical property and physical properties are controlled by intermolecular forces. The stronger the intermolecular forces, the more the molecules are attracted to each other, and more energy needs to be put in to separate them from each other. This would result in a higher boiling point. Diethyl ether can't form H bonds and only has weaker dipole-dipole forces. The alcohols have can form H bonds due to the presence of the H atoms attached to the electronegative O atoms. H bonds are the strongest intermolecular forces so the alcohols have higher boiling points than the ether.
e. Stability trends are controlled by intramolecular forces, the covalent bonds that hold the atoms in the molecules together. Boiling point and other physical properties are controlled by the much weaker intermolecular forces.
Common errors:
b. Poorly drawn energy diagrams that failed to show the connection between the isomers i.e. that they combustion to give the same mixture of carbon dioxide and water. The "most stable" isomer circled did not correspond to the lowest energy structure.
c. Not rationalising stability in terms of intramolecular forces (internal bond strengths).