a.
Part
6: THERMODYNAMICS
a.

b.
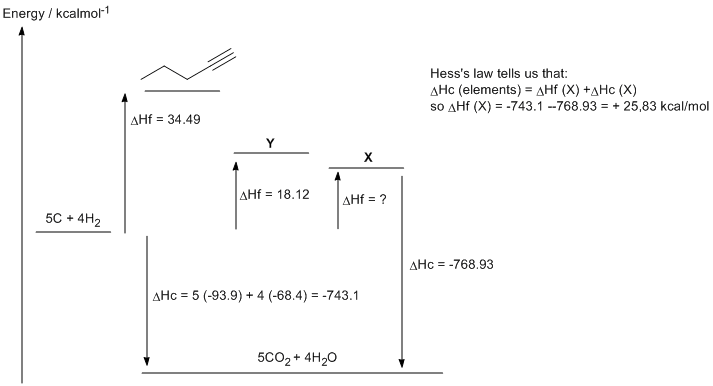
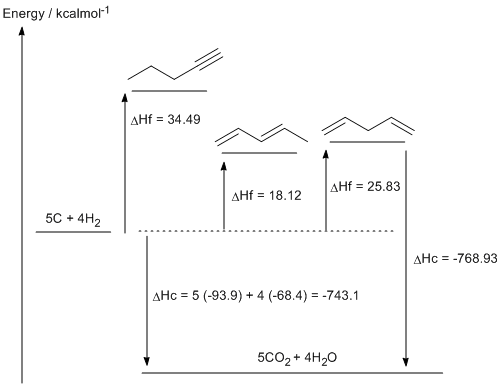
c. 1,3-pentadiene (DHf = 18.12 kcal/mol) is more stable that 1,4-pentadiene (DHf = 25.83 kcal/mol) due to the resonance type interaction that is possible between the two double bonds. This adds double bond character to the single bond between the two double bonds.
d.
Common errors:
a. Not drawing the alkyne correctly, Alkynes have linear geometry and need to be drawn that way!
c. Did not recognise that resonance interaction of the adjacent C=C in 1,3-pentadiene would add to the stability of that system. Instead, arguments based on symmetry (no idea what that is based on!), electron cloud repulsion, branching differences (they are the same?). The common misconception that bonds contain energy was also used and therefore the diagram was draw upside down! : (