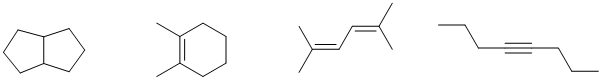
Part
5: STRUCTURE DETERMINATION
a. For a hydrocarbon, the formula for the index of hydrogen defficiency, IHD = 0.5 ( 2c+2-h), therefore, the IHD = 0.5 (2 x 8 + 2 -14) = 2
FYI : The IHD = pi + r (pi bonds plus rings)
there can be 2 units of unsaturation, which could mean 2 rings, a ring and a double bond, two double bonds or a triple bond.
b. Here are possible answers (there are likely others), these were selected to represent four different ways to get an IHD=2. Note that there has to be some symmetry.
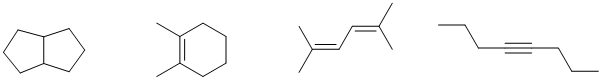
c. The structure shown below actually has two E double bonds (the higher priority alkyl groups need to be on opposite sides of the C=C).
![]()
d. The IR data 2150 cm-1 implies an alkyne, the 3300 cm-1 implies an sp C-H, i.e. terminal alkyne. A generic example and a specific example are shown below. Note that alkyne geometry in linear.
![]()
e.
i. The terminal alkyne will be the most acidic choice, pKa about 25.
ii The conjugate base of an alkyne has the -ve charge associated with an sp hybrid orbital. This means the electrons are in a small orbital (due to the high s character) that puts the -ve charge closer to the positive nucleus. This stabilises the conjugate base. (i.e. think of HA <=> H+ and A-, stablising A- favours the right hand side indicating that HA is a stronger acid).
iii. A generic balanced equation for the reaction of a terminal alkyne as an acid is shown.:
![]()
f.
i. The reaction will be allylic radical bromination. This is the expected product because allylic C-H bonds are the weakest and this leads to a resonance stablised radical.
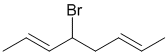
ii. Comparing the initial molecular formula with C8H14Br2 indicates that an addition reaction has occurred (addition to a C=C as per the hydrocarbon experiment from the laboratory), likely if a high concentration of bromine was used (in error).
Common errors:
Incorrectly counting the types of H.
Ignoring the fact that the question indicates that X was a hydrocarbon.
Did not recognise the terminal alkyne in part d.
Did not use a strong enough base (pKa >25) to deprotonate the terminal alkyne.
Confused the pKa of internal and terminal alkynes.
Drawing vinyl bromides (C=C-Br) as products of radical bromination (it's a reaction of sp3 C-H bonds).
Not recognising the electrophilic addition in part fii.