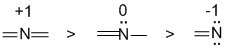
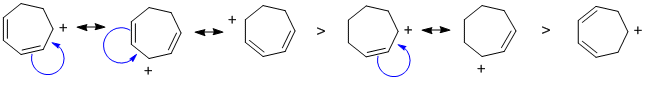
Qu 4:
| Qu1: | Assign the formal charges...Nitrogen with 4 bonds is +1, with 3 bonds and 1 lone pair is 0 (neutral) and with 2 bonds and 2 lone pairs is -1 | 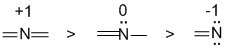 |
| Qu 2: | All the cations are secondary , the difference is the resonance stablisation. More resonance contributors means greater stability. | 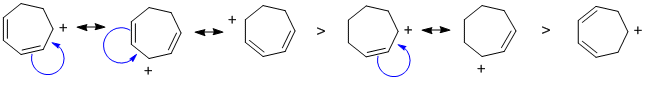 |
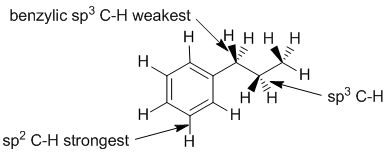
| Qu 3: | C-H bond strength depends on the hybridisation of the C atom. sp2 hybrid orbitals have more s character and so are smaller which leads to shorter, stronger bonds. The proximity of a p system (here a benzene rings) makes adjacent C-H bonds weaker (the e rich character of the p system allows the adjacent C-H bonds to lengthen and therefore weaken). |  |
Qu 4: |
Basicity...either think about the availability of the electrons in the base or the stability of the bases. | |
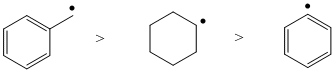
| Qu 5: | The hydrocarbon reactivity experiment helps answer this question. Resonance can stabilise radicals. |  |
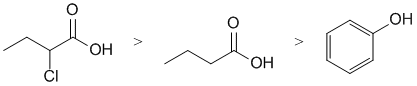
| Qu 6: | Carboxylic acida (pKa about 5) are more acidic than phenols (pKa about 10) due to the extra resonance stabilisation of the carboxylate conjugate base. The Cl atom adjacent to the C=O group further stabilises the conjugate base (due to inductive effects caused by the Cl electronegativity) which means that the Cl substituted acid is the strongest acid. |  |
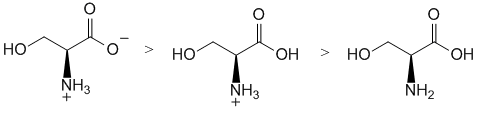
| Qu 7: | At a pH of 2.5, the carboxylic acid (pKa given as 2.12) will be mainly deprotonated (pH is more basic than the pKa) but the amine will still be protonated (pKa given as 9.15). The structure with the carboxylic acid and the free amine can't really exist in solution as the acid is acidic enough to protonate the amine. |  |
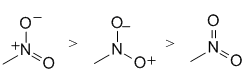
| Qu 8: | Ranking resonance structures... Can't exceed the octet on N, but maximise the number of atoms with complete octets and minimise charge separation. |  |
Qu9: The structure contains 3 rings, 4 C=C and 3 C=O (two amides and a carboxylic acid) therefore index of hydrogen defficiency = 10.
Qu10: The key to basicity is electron availability. Since C2 has no lone pairs is is not very basic. Now use periodic trends.... basicity decreases as you go down a group in the periodic table so that means we need to consider O9, N10 and N14. Due to electronegativity, O is less basic than N. If we compare N10 and N14 we see that N10 is part of an amide and so the N lone pair is less available due to it being involved in resonance with the C=O. So N14 is the most basic.
Qu11: For C6 assign the R/S stereochemistry, using the Cahn-Ingold-Prelog rules to assign group priorities....N > C-N > C-C > H. Fortunately the lowest priority group, the H is already at the back, so the since the sense of the 3 higher priority groups (1-2-3) is clockwise = R. For C2=C3 assign E or Z to the alkene based on the priority rules at each end of the C=C unit. At C2, N > CO and at C3 Cl > C, therefore the higher priority groups are on opposite sides = E.
Qu12: Oxidation states...count the bonds attached to the atom being considered. A bond to a more electronegative atoms counts -1, a bond to the same type of atom counts 0 and a bond to a less electronegative atom (e.g. H) counts +1. Total the count and then consider the formal charge on the central atom since the oxidation state for the central atom plus the groups attached must equal the atoms formal charge. For C13 the C is attached to 2 x C (count 0 each), (count -1) and H (count +1) therefore total = 0 and therefore the oxidation state C = 0.
Qu13: Functional groups....the group in the box is a N-C=O unit, an amide.
Qu14: Single bonds are longer than double bonds, larger atoms form longer bonds and since sp3 hybrid orbitals are larger than sp2 hybrid orbitals (due to more p character), the longest bond is the single bond between the 2 sp3 C atoms, C4-C5.
Qu15: N10 is part of an amide so the lone pair is in a p orbital to allow the favourable resonance interaction with the adjacent C=O group.
Qu16: C4 has two C groups and two H groups attached so its is sp3. N14 has one C, 2 H and a lone pair, and therefore since there is no adjacent pi system (and hence no resonance), the N is sp3.
Qu17: C13 is attached to two C atoms so it is secondary. It is also adjacent to a benzene ring so it is benzylic.
SPECTROSCOPY:
Use the
IR spectra provided to get the functional groups that are present in the structures, so look for the
key groups : C=O (near 1700 cm-1) , -OH or -NH (above
3000 cm-1), aromatic C=C (two bands 1600-1400
cm-1), C-O (near 1200
cm-1) and triple bonds (near 2200
cm-1).... use the tables as needed.
Qu18: The IR shows a NH2 stretch at 3300 cm-1, no C=O near 1700 cm-1 (look for a primary amine) and the C-H stretches are < 3000 cm-1 indicating sp3 C-H only. The only amine in the list is cyclohexylamine.
Qu19: The IR shows a strong and broad OH stretch at 2500-3500 cm-1, and C=O near 1700 cm-1 (look for a carboxylic acid) but no Ar C=C near 1600 cm-1. Therefore the IR is of butanoic acid.
Qu20: The IR shows a C=O near 1700 cm-1. The IR does not show OH or NH. On closer inspection, the C-H stretches are < 3000 cm-1 indicating sp3 C-H only and there is no Ar C=C near 1600 cm-1, so it must be cyclohexanone.
Qu21: The IR does not show OH, NH, C=O or CN triple bond. In the CH region, the IR also shows both sp3 and sp2 CH (either side of 3000 cm-1), therefore cyclohexene.
Qu22: The IR does not show OH, NH, C=O or CN triple bond. In the CH region, the IR also shows only sp3 CH < 3000 cm-1, therefore cyclohexane.
Qu23: The IR shows a C=O just below 1700 cm-1, and Ar C=C near 1600 cm-1 . There is no OH or NH near 3500 cm-1, therefore the aromatic ketone, acetophenone.
NOMENCLATURE:
Two types of questions here, however it is a good idea to use key descriptors like E and Z, R or S,
cis or
trans, numbering, functional group etc. to help screen the answers before getting too tied up in the words. For the first four, name the compound and then match it to the list, for the last four
use the key descriptors and check each molecule in turn. So this section really depends on the
nomenclature rules.
Qu24: An alkane with the longest chain C8, so an octane. There are two substituents, a simple ethyl group and a complex substituent, a 1,1-dimethylethyl group. These need to be numbered 4,5 and alphabetisation means that the dimethylethyl group is named before the ethyl group since the first letter in the brackets is used from complex substituents when alphabetising such systems. Hence, 4-(1,1-dimethylethyl)-5-ethyloctane.
Qu25: The longest chain is C5, so a pentane. The principle functional group is a ketone, so the suffix "-one" is required. There is also an alcohol that needs to be treated as a substituent (hydroxy). Number to give the ketone the lowest number means 5-hydroxypentan-2-one.
Qu26: The principle functional group is the alkene and those two C atoms need to be 1 and 2. Then the first point of difference rule requires that the system be numbered as a 1,6,6 trimethyl system rather than 2,3,3-, so this means we have 1,6,6-trimethylcyclohexene.
Qu27: The longest chain is C6, with an alkene as the principle functional group so a hexene. Need to number to give the alkene the lowest number, i.e. -3-ene. We have an ethyl substituent which needs to be numbered as 3- rather than 4- (alphabetisation) and a methyl group at C4 . Since the 2 groups attached to the C3 ebd of the alkene are the same, there is no cis/trans or E/Z stereochemistry. Hence we have 3-ethyl-4-methylhex-3-ene.
Qu28:
|
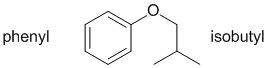 |
|
Qu29:
|
Substituted benzenes | 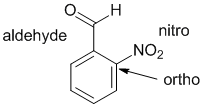 |
Qu30:
|
R/S stereochemistry | 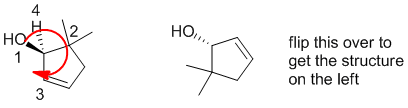 |
Qu31:
|
See polycyclic systems.
|
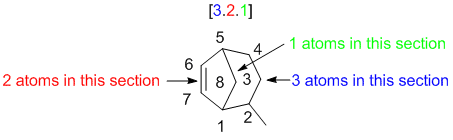 |