i. The structure needs to contain a ring and either an alkene or an alkyne. The reaction would be an addition.
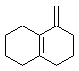
a. Index of hydrogen deficiencies = IHD = 4 (this helps us know that the structures must contain 4 units of unsaturation based on triple bonds, double bonds, or rings).
b.
i. The structure needs to contain a ring and either an alkene or an alkyne. The reaction would be an addition.

ii. The structure needs to be a terminal alkyne. Terminal alkynes are more acidic because the negatove charge in the conjugate base is in an sp hybrid orbital where the high %s character (50%) puts the charge close to the positive nucleus.
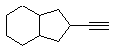
c. Techniques such as IR (look for C=C and / or CC bonds and / or sp / sp2 CH) or 13 C NMR (look for sp2 C or sp C) or H NMR (look for vinyl H (C=C-H) vs alkyne C-H).
d.
i.& ii.
![]()
iii.
# mol of C11H16 = 1.00 g / (148.25 g/mol) = 6.745 x 10-3 mol C11H16 = max amount of C11H15Br possible = theoretical yield
MW of C11H15Br = 227.14g/mol
Theoretical yield = 6.745 x 10-3 mol x 227.14 g/mol = 1.53 g
% yield = (actual yield / theoretical yield) x 100% = (0.80 g/ 1.53 g) x 100% = 52%
General: Drawing structures that did not match the molecular formula, or did not contain the right functional groups.