Part 7: MECHANISM
The following diagram shows the solution to the
mechanistic question. Note that all the information applies to a single
reaction sequence that has been completely described verbally.
There is no need for extra reagents or
extra steps etc. The curly arrows are drawn specifically to match the
text in the question. The biggest problem students have is making sure they
understand the language of chemistry. Most students have trouble because they
can't draw the structures from the IUPAC names (that means they don't know their
nomenclature well enough). Read the words carefully, and then make the curly
arrows tell that same story. There is NO need for extra steps. Remember
curly arrows go from electron rich to poor and to balance the
formal charges at each step - errors on formal charges were common.
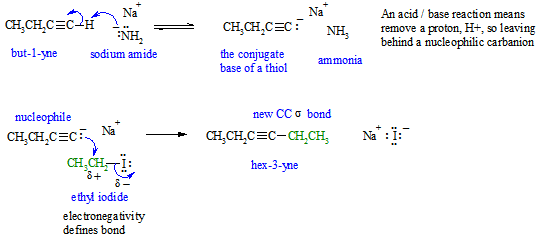
a.

If you struggled with this part of the question, first draw the compounds whose names were provided, then think about the types of reactions (e.g. acid / base) and try to fill in the structures in the gaps, then finally add the required curly arrows to account for all the bonding changes.
b. Since the reaction requires deprotonation of a terminal alkyne (pKa about 25) we need quite a strong base (i.e. pKa > 25). A reasonable example would be a alkyl carbanion such as CH3- (pKa > 50). Note that the amide ion (NH2-) used in the paragraph has a pKa about 35.
c.
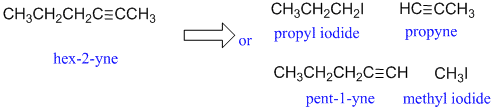
d.
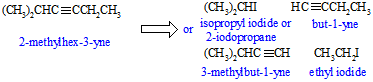
e. In the analogous reaction of but-1-ene with ethyl iodide, sodium amide (pKa = 35) is not a strong enough base to remove a proton from an sp2 C-H bond in the alkene (pKa = 45), thus one can not prepare the required nucleophile for the reaction to work unless you were to use a stronger base.
Common errors:
a.
b. Poor knowledge of acids and bases e.g. suggesting things like HO- , RO- , H2O etc or worst , H2SO4
c & d. Did not use part a as the basis of working out the answer. Only showing one component, instead of simply drawing a structure trying to name a compound and not doing it correctly (i.e. poor nomenclature). Not taking into account the number of C atoms that were being combined. c. Suggesting but-2-yne but there is no terminal alkyne C-H to be removed there. Suggesting but-3-yne (incorrect naming).
d. Suggesting 2-methylbut-1-yne.... an impossible structure
e. Poor knowledge of acidity. Rationale based on pKa alone, incomplete rationale. Answering a different question because they did not read the question asked carefully enough and therefore made avoidable errors including talking about the triple bond in a butene (that's an alkene). Talking about radical stability or C-H bond strengths... since the reaction does not involve radicals, they are irrelevant. Bond strength is not a good predictor of acidity.