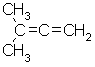
a. Elemental analysis : Since the % add up to 100% we know we have a hydrocarbon. The question tells us the mass spectrum showed the molecular ion at m/z =68, so the MW=68 g/mol. Hence one can calculate the empirical formula = C5H8
b. In this problem, the molecular formula equals the empirical formula.
c. Index of hydrogen deficiencies = IHD = 2 (this helps us know that the structures need below must contain either a triple bond, 2 double bonds, 1 double bond and a ring or 2 rings).
| d. | 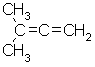 |
e. | f. |
Common errors:
General: Drawing structures that did not match the molecular formula.
a . Could not do elemental analysis calculations.