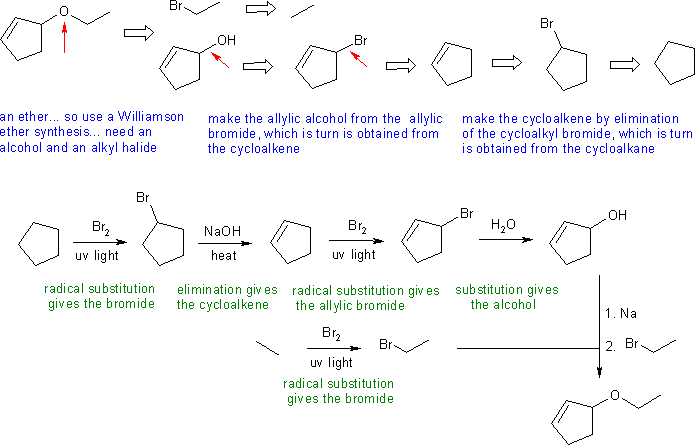
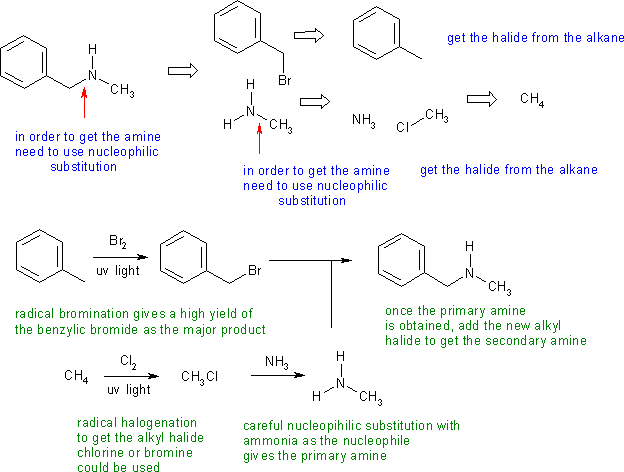
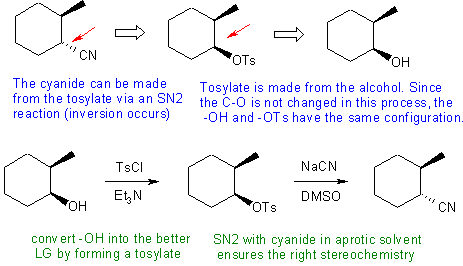
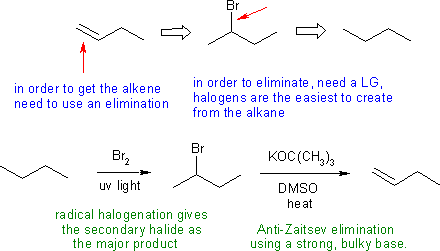
Answers to the synthesis problems are given below. These answers have been selected as they are short but there are probably other reasonable / alternative solutions ..... Of course they are based on the reactions covered in the Fall semester !
Common errrors:
A: Selecting routes that gave poor control on the location of the double bond.
B: Incorrect reagents for adding the Br to the toluene to make a substrate for SN reactions. Failing to show how methyl amine, CH3NH2 is prepared.
C: Ignoring the stereochemistry or getting the stereochemistry wrong by attempting a double inversion when only one is needed. We also need to avoid SN1 conditions and the associated carbocation to avoid any rearrangements and loss of stereochemistry - this means no using an acidic route to converting the -OH to a better leaving group.
D: Introducing a halogen into the 1-position - both Cl2 and Br2 give the 2-halobutane as the major product. Using hydroxide to eliminate the 2-halobutane will give the 2-butene as the major product. Routes via 2-butanol will give trans-2-butene as the major product.



