Part 9 : STRUCTURE DETERMINATION
First, the flow chart of the basic
information from the question.... use this to try to answer the
question before just looking at the answers....
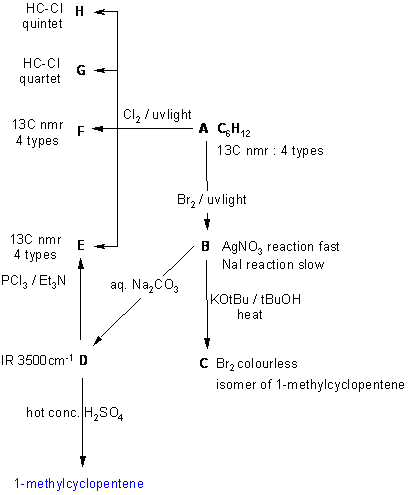
A schematic of the solution is shown
below. The information from the
question is given in black. Deductions directly from this
information
are given in red. Points that
provide
potential key information are shown by green arrows
which leads to the structures which are linked via the blue
arrows to show the path required to work them all out. There are, of
course,
other possible thought pathways.
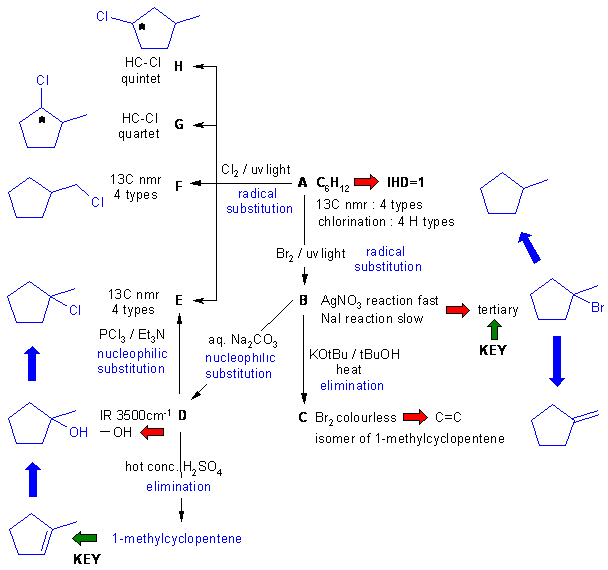
Important points:
(1) D gives
1-methylcyclopentene ... hence we have a
methylcyclopentane framework.
(2) A has 4 types of C
and H, one of which is tertiary.
(2) B is a tertiary
bromide - evident from synthesis from A and reactions with AgNO3 and NaI
(3) Elimination of B with
a hindered base gives the less substituted alkene as the major product
(4) Dehydration of alcohol D
gives the more stable, more highly substituted alkene as the major
product
(5) F, G and H can de deduced once A is obtained.
(6) No carbocation rearrangements since B and D are already tertiary


