| GEOMETRIC isomers....
(C=C with cis / trans or E / Z isomers) has to be E-1-chloropropene and Z-1-chloropropene. |
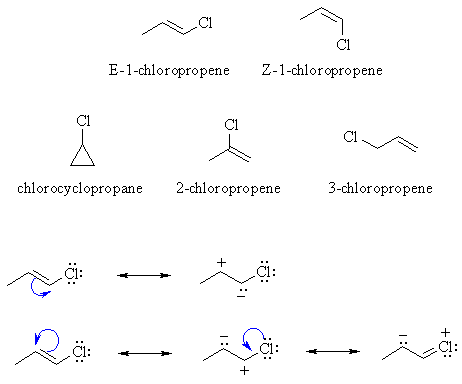 |
| CONSTITUTIONAL isomers .... there are 3 other isomers, chlorocyclopropane, 2-chloropropene and 3-chloropropene. | |
| RESONANCE.....Only those structures with p bonds can exhibit resonance, so there can be no resonance in the cyclopropane. However all those with p bonds can exhibit resonance we have a couple of general possibilities that are shown above. Note that in principle, the lone pairs on the Cl atom could be used to stabilise an adjacent +ve charge. |