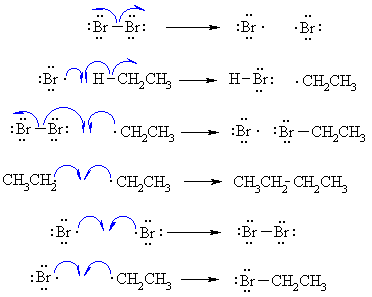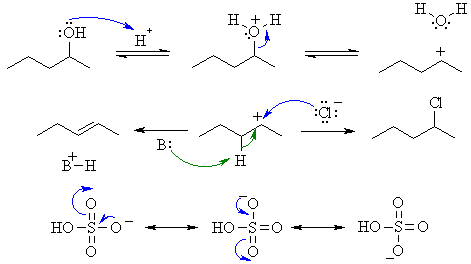
I
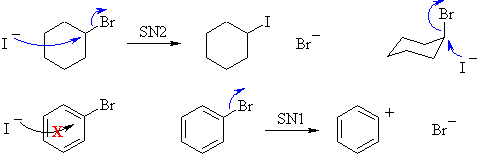
Alkyl bromides react with sodium iodide in acetone to produce an alkyl
iodide via an SN2 type of reaction (strong nucleophile, polar aprotic
solvent).
However aryl bromides do not undergo nucleophilic substitution
reactions.
The planar geometry of the ring prevents the backside attack (180o)
and an SN2 reaction. The SN1 type would require the formation of
a phenyl cation which is an unfavourable carbocation, between primary
and
methyl in stability, making it unfavourable. Plus the aromatic
ring
is already electron rich so it not usually attacked by nucleophiles.

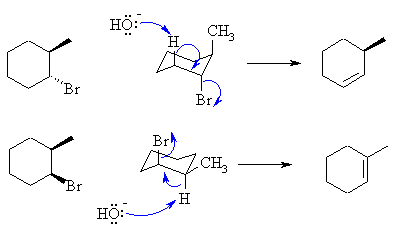
| These reactions are E2 reactions since we have a
secondary
bromide in the presence of a strong base. The E2 reaction is a
concerted
process that requires the H and LG to be at 180o
(antiperiplanar).
In cyclohexanes, this requires that both the Br and the H that is
removed
be in axial positions.
In the trans-isomer, elimination to the less stable
alkene occurs
since the Me group is also in the axial position.
|
 |