 |
|
 |
|
According to the IUPAC rules, locants can be omitted if:
(note that some of these conditions overlap with each other).
Here are some illustrative examples that try to capture these implications of this at this "introductory organic chemistry" level:
| ethanol | Locant dropped | unambiguous, all C atoms in the parent chain (ethane) are equivalent | |
| propene | Locant dropped | unambiguous, C=C must be at the end of the parent chain | |
| propan-1-ol | 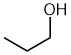 |
Locant can not be dropped | ambiguous, -OH group could be on C1 or C2 of the parent chain (propane) which are different |
| propanal | 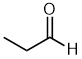 |
Locant dropped | unambiguous, an aldehyde must be at the end of the chain which defines C1 |
| propan-2-one |  |
Locant can not be dropped | Ketones aren't restricted to ends of chains and the parent chain (propane) has two different positions. (IUPAC specifically cites this example) |
| cyclohexanol | 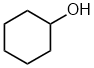 |
Locant dropped | unambiguous, all positions on the parent ring (cyclohexane) are equivalent |
| butan-2-one |
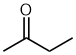 |
Locant can not be dropped | Ketones aren't restricted to ends of chains and the parent chain (butane) has two different positions (IUPAC specifically cites this example) |
| 2-chloroethan-1-ol | 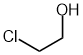 |
Locants can not be dropped | View ethanol as the parent, then there are two places the substituent could be and therefore required to include both locants. (IUPAC specifically cites this example) |
| cyclohexane-1,2-diol | 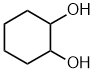 |
Locants can not be dropped | With two groups on a cyclic structure both locants are required according to the IUPAC rules to distinguish 1,1-, 1,2-, 1,3- etc. |
| prop-2-en-1-ol | Locants can not be dropped | With two groups on parent structure both locants are required according to the IUPAC rules to distinguish 1,1- & 1,2-. | |
| cyclohex-2-en-1-ol | 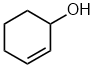 |
Locants can not be dropped | With two groups on a cyclic structure both locants are required according to the IUPAC rules to distinguish 1,1-, 1,3-, 1,4- etc. |
| 3-bromocyclohex-1-ene | 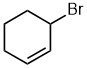 |
Locants can not be dropped | With two groups on a cyclic structure both locants are required according to the IUPAC rules to distinguish 1,1-, 1,3-, 1,4- etc. (IUPAC specifically cites this example) |
| ©Dr. Ian Hunt, Department of Chemistry |