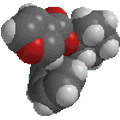
Haloalkane
style:
- Functional group is an alkane, therefore suffix
= -ane
- The longest continuous chain is C2 therefore
root = eth
- The substituent is a bromine, therefore prefix
= bromo
- No locant is required since the -Br location is unambiguous (i.e. substitution at either carbon gives the same molecule)
bromoethane
Alkyl
halide style:
- The alkyl group is C2, it's an ethyl
- The halogen is a bromine, therefore suffix = bromide
ethyl bromide |
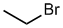
CH3CH2Br |
|
Haloalkane
style:
- Functional group is an alkane, therefore suffix
= -ane
- The longest continuous chain is C3 therefore
root = prop
- The substituent is a chlorine, therefore prefix
= chloro
- The first point of difference rule requires numbering
from the right as drawn,
the substituent locant is 1-
- The locant is required to distinguish between 1- and 2-chloropropane
1-chloropropane
|
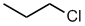
CH3CH2CH2Cl |
|
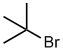
Alkyl
halide style:
- The alkyl group is C4, it's a tert-butyl
- The halogen is a bromine, therefore suffix =
bromide
tert-butyl bromide
Haloalkane style:
- Functional group is an alkane, therefore
suffix = -ane
- The longest continuous chain is C3 therefore
root = prop
- The substituent is a bromine, therefore prefix
= bromo
- There is a C1 substituent = methyl
- The substituent locants are both 2-
2-bromo-2-methylpropane
|
(CH3)3CBr
|
|
Haloalkane style:
- Functional group is an alkene, therefore suffix
= -ene
- The longest continuous chain is C4 therefore
root = but
- The substituent is a bromine, therefore prefix
= bromo
- Since bromine is named as a substituent, the
alkene gets priority
- The first point of difference rule requires numbering from the left
as drawn to make the alkene group locant 1-
- Therefore the bromine locant 4-
4-bromobut-1-ene
|
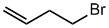
CH2=CHCH2CH2Br
|
|