Qu 1:
|
By drawing the two amide C=N resonance contributions we
can get a benzenoid structure with aromatic character (cyclic, planar,
conjugated with 4n + 2 π electrons) |
|
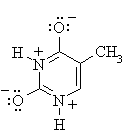
|
|
|
| Qu 2: |
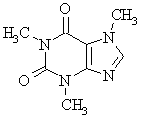
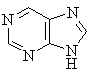
Caffeine is a purine derivative as can be seen by comparing the two
structures shown below: |
|
 |
 |
|
caffeine
|
purine
|
|
|
|
| Qu 3: |
A doublet code would only give 42 = 16 combinations which
is insufficient for the 20 amino acids. |
|
A triplet code gives 43 = 64 combinations, more than
enough ! |
|
|
| Qu 4: |
You need to recognise the structural units in order to answer these
questions.... go back and review them if you can't ! |
|
(a) 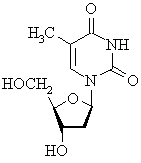 |
This nucleoside (no phosphate ester) has the pyrimidine base thymine
(T) base, a deoxyribose carbohydrate (note the missing 2'-hydroxy group,
hence deoxy-) and is therefore from the DNA series. |
(b) 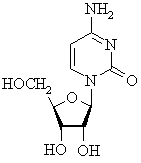 |
This nucleoside (no phosphate ester) has a cytosine (C) base, a ribose
carbohydrate, and is therefore from the RNA series. |
(c) 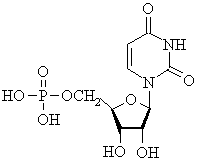 |
This nucleotide (note the phosphate ester group) has a uracil (T) base,
a ribose carbohydrate, and is therefore from the RNA series |
|
|
|
