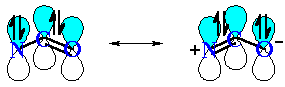-
The resonance interaction of the N lone pair with the C=O of
the carbonyl in the amide results in the C-N bond having some double
bond character (shorter, restricted rotation)
-
The planar sp2 N system allows the N lone pair to align with
the C=O π system (see image below, with
the other bonds omitted for clarity)
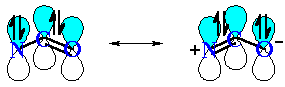
-
This has structural implications even in simple amides.
- Look at the amide
bonds in the JSMOL images to the right.
-
What do you notice about the geometry at the N atoms ? ANSWER
Implications
-
Structure :
-
The double bond character of the C-N unit makes the structure less
flexible.
-
Planarity and the anti-conformation are important in dictating protein
shape.
-
Reactivity :
-
The resonance donation of electrons by the N makes the carbonyl less electrophilic.
-
As a result the amide is comparatively unreactive.
This is a good thing otherwise proteins would be too reactive to be
much use in biological systems !
|
the simplest amide
dipeptide Gly-Ala
dipeptide Ala-Gly
|