| Chapter 24: Phenols |
Carboxylation of Phenols (Kolbe-Schmitt reaction)
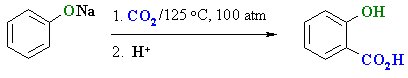
Reaction type: Electrophilic Aromatic Substitution
Summary
- Heating the nucleophilic phenolate salt with carbon dioxide under high pressure / temperature results in regioselective ortho-substitution.
- This process is also known as the Kolbe-Schmitt synthesis.
- o-hydroxybenzoic acid is more commonly known as salicyclic acid.
 |
|
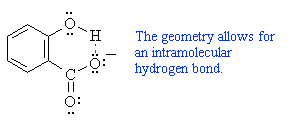
| Check out the CHIME image for the intramolecular hydrogen bond by looking the the postion of the shared H atom with respect to the two oxygen atoms |
QUESTIONS
| Study Tip:
Consider the phenolate to be an enolate, hence reactions at the a-C are typically favoured. |
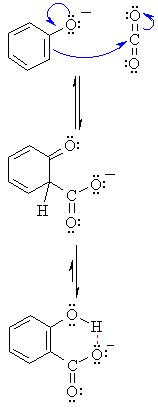
MECHANISM FOR CARBOXYLATION OF PHENOLS Step 1:
The nucleophilic phenolate (reacting like an enolate) reacts with the electrophilic carbon of carbon dioxide in the ortho position (compare this with an Aldol reaction)Step 2:
The non-aromatic cyclohexadienonecarboxylate intermediate tautomerises to the more stable aromatic enol which is further stabilised by an intramolecular hydrogen bond. An acidic work-up will generate the carboxylic acid.
| © Dr. Ian Hunt, Department of Chemistry |