 |
Chapter 24: Phenols |
 |
Phenols
Nomenclature:
Functional group suffix = -common - phenol, systematic - benzenol
(review)
Functional group prefix = hydroxy
Numbering of the ring begins at the hydroxyl-substituted carbon and
proceeds in the direction of the next substituted carbon that possesses
the lower number.
Ortho, meta or para ? Mono-substituted phenols are characterised
using the prefix ortho (o-), meta (m-) or para (p-)
depending on the placement of the substituent from the hydroxyl group or
the hydroxyl group from a higher priority functional group, 1,2-, 1,3-
or 1,4- respectively.
|
|
|
|
1,2-chlorophenol
or
o-chlorophenol
|
1,3-chlorophenol
or
m-chlorophenol
|
1,4-chlorophenol
or
p-chlorophenol
|
There are several other functional group suffixes, for various substituted
phenols, that one needs to know, check here.
Physical Properties:
-
The polar nature of the O-H bond (due to the electronegativity difference
of the atoms ) results in the formation of hydrogen bonds with other
phenol molecules or other H-bonding systems (e.g. water). The implications
of this are:
-
high melting and boiling points compared to analogous arenes
-
high solubility in aqueous media
-
The presence of intramolecular hydrogen bonding is believed responsible
for the significantly lower boiling points of certain ortho-substituted
phenols vs the meta- and para- analogs.
Structure:
|
|
-
The alcohol functional group consists of an O atom bonded to an
sp2-hybridised aromatic C atom and a H atom via
σ
bonds.
-
Both the C-O and the O-H bonds are polar due to the high
electronegativity of the O atom.
-
Conjugation exists between an unshared electron pair on the oxygen and
the aromatic ring.
-
This results in, compared to simple alcohols:
-
a shorter carbon-oxygen bond distance
-
a more basic hydroxyl oxygen
-
a more acidic hydroxyl proton (-OH)
|
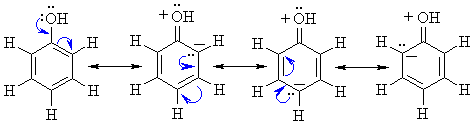
Acidity:
-
Phenols are more acidic (pKa»10)
than alcohols (pKa»16 - 20),
but less acidic than carboxylic acids (pKa»5)
-
The negative charge of the phenolate ion is stabilised by resonance due
to electron delocalisation onto the ring as shown below:
-
The acidity difference means that it is possible to separate phenols
from alcohols and/or carboxylic acids.
-
Mixing an ether solution, of either phenol and alcohol or phenol and carboxylic
acid, with dilute base (sodium hydroxide and sodium bicarbonate, respectively),
results in the stronger acid being converted to its alkali salt, which
is then extracted to the aqueous phase and can be separated from the organic
phase.
-
Nucleophilic substitution reactions of phenols are generally carried
out under basic conditions as the phenolate ion is a better nucleophile.
Substituent Effects on Acidity
Substituents, particularly those located ortho or para
to the -OH group, can dramatically influence the acidity of the phenol
due to resonance and / or inductive effects. Electron withdrawing groups
enhance the acidity, electron donating substituents decrease the acidity.
The resonance stabilisation of o-nitrophenol is shown below:
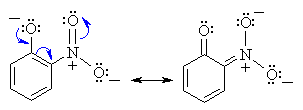
|
Compound
|
pKa |
|
Compound
|
pKa |
|
Phenol
|
10.0 |
|
|
|
|
o-Methoxyphenol
|
10.0 |
|
p-Methoxyphenol
|
10.2 |
|
o-Methylphenol
|
10.3 |
|
p-Methylphenol
|
10.3 |
|
o-Chlorophenol
|
8.6 |
|
p-Chlorophenol
|
9.4 |
|
o-Nitrophenol
|
7.2 |
|
p-Nitrophenol
|
7.2 |
|
m-Nitrophenol
|
8.4 |
|
|
|
QUESTIONS
-
Draw resonance structures to show the stabilisation of p-nitrophenol. ANSWER
-
How about m-nitrophenol ? ANSWER
Study Tip:
Did you answer question about the m-nitrophenol correctly ? If you
did, you probably have a good understanding of resonance and curly arrows.
If not, you may want to review resonance
and curly arrows again. |
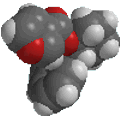
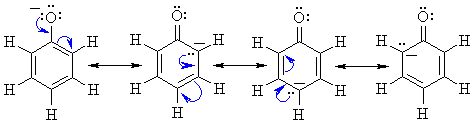
Reactivity:
 |
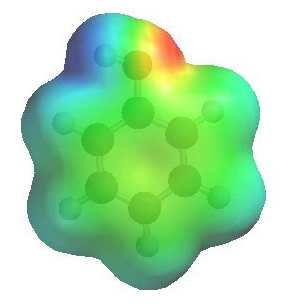
The image to the left shows the electrostatic potential for phenol.
The more red an area is, the higher
the electron density and the more blue
an area is, the lower the electron density.
-
The hydroxyl O atom is a region of high electron density (red)
due to the lone pairs.
-
The hydroxyl O atom can function as a nucleophile or Lewis base.
-
There is low electron density (blue)
on H atom of the hydroxyl group, i.e. H+ character, therefore
phenols are acidic (pKa ~ 10)
-
Due to conjugation with the ring, phenols are more acidic than alcohols
(pKa ~ 16).
-
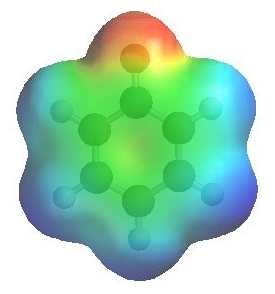
Removal of the proton generates a phenolate ion.
The image to the left shows the electrostatic potential for a phenolate
ion.
The more red an area is, the higher
the electron density and the more blue
an area is, the lower the electron density.
-
Note the increased electron density on the oxygen compared to the phenol
|




