| Chapter 23: Aryl Halides |
Nucleophilic Aromatic Mechanism
As we have already seen (chapter 12), the most common reaction of aromatic systems is electrophilic aromatic substitution :
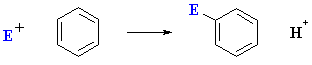
However, a fundamentally different type of reaction is possible for aryl halides under certain conditions:
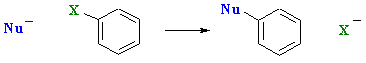
The fundamental requirements for this nucleophilic aromatic substitution reaction are:
- ortho- or para- nitro group or groups, and strong nucleophile, e.g. CH3O-
- or a very strong nucleophile, e.g. NH2-
1. formation of the new σ bond
from an Ar C to nucleophile
2. breaking of the σ bond to
the leaving group
Depending on the relative timing of these two events, two mechanistic pathways emerge:
- Addition-Elimination (add Nu, loose LG)
- Elimination-Addition (loose LG add Nu)
| © Dr. Ian Hunt, Department of Chemistry |