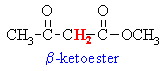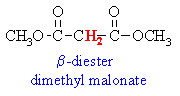|
Chapter 21: Ester
Enolates |
 |
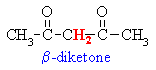
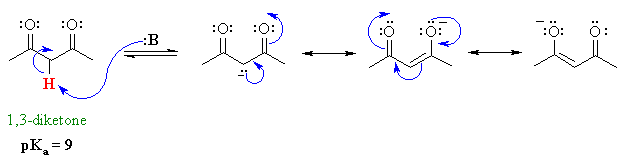
Active Methylenes
- In some molecules there are H
atoms that are adjacent to 2 carbonyl groups (which are electron
withdrawing).
- Extra resonance stabilisation
of the enolate anion makes these H more acidic (i.e lower pKa)
than hydrogens adjacent to a single carbonyl group.
- These type of compounds are sometimes
called "active methylenes" (recall a methylene group is
a CH2)
Some common active methylene compounds
are shown below as JMOL images... make sure you can see the acidic active methylene
hydrogen atoms...
- As we will see later
is this Chapter, these compounds are useful synthetic intermediates.
- Their enolates can be formed
readily and can be alkylated, then further manipulated.