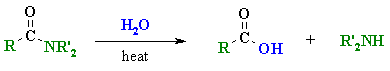|
MECHANISM
OF THE ACID CATALYSED HYDROLYSIS OF AMIDES
|
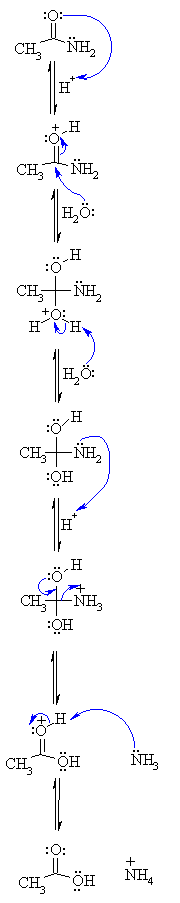
Step 1:
An acid/base reaction. Since we only have a weak nucleophile and apoor
electrophile we need to activate the amide. Protonation of the amidecarbonyl
makes it more electrophilic. |
|
Step 2:
The water O functions as the nucleophile attacking the electrophilicCin
the C=O, with the electrons moving towards the oxonium ion, creatingthe
tetrahedral intermediate. |
Step 3:
An acid/base reaction. Deprotonate the oxygen that came from the watermolecule
to neutralise the charge. |
Step 4:
An acid/base reaction. Need to make the -NH2leave, but
need to convert it into a good leaving group first byprotonation. |
Step 5:
Use the electrons of an adjacent oxygen to help "push out" the leavinggroup,
a neutral ammonia molecule. |
Step 6:
An acid/base reaction. Deprotonation of the oxonium ion reveals thecarbonyl
in the carboxylic acid product and regenerates the acid catalyst. |
| |