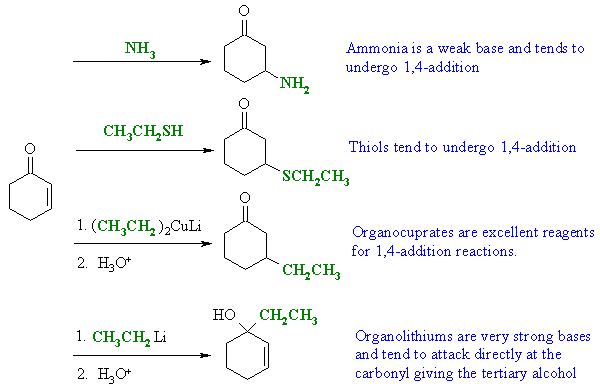| Qu1: |
How many α-hydrogens
are there in each of the following aldehydes and ketones : |
| |
| (a) (CH3)2C=O
= 6 (2 x CH3) |
| (b) CH3CHO
= 3 (1 x CH3) |
| (c) H2C=O
= 0 (the 2H are attached directly to the carbonyl,
not the adjacent a-position) |
| (d) PhCHO = 0
(there are no H on the adjacent a-C which
is a substituted aromatic carbon) |
| (e) 2-pentanone = CH3C=OCH2CH2CH3
= 5 (1 x CH3 plus 1 x CH2) |
| (f) 3-pentanone
= CH3CH2C=OCH2CH3
= 4 (2 x CH2) |
|
| Qu 2: |
|
| |
|
| |
|
| Qu 3: |
|
| |
|
MECHANISM OF ACID
CATALYSED TAUTOMERISATION
|
|
Step 1:
First, an acid-base reaction. The Lewis basic O atom of the carbonyl
is protonated by the acid catalyst giving an oxonium ion. |
 |
Step 2:
Another acid-base reaction. Removal of an α-hydrogen
by a water molecule functioning as a base allows formation of the
C=C and neutralises the positive charge on the O giving the enol
and regenerates the catalyst. |
|
|
MECHANISM OF BASE
CATALYSED TAUTOMERISATION
|
|
Step 1:
First, an acid-base reaction. Hydroxide functions as a base and
removes the acidic α-hydrogen giving
the reactive enolate. |
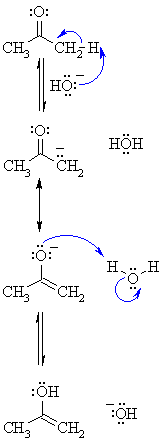 |
Step 2:
The negative charge is resonance stabilised to the more electronegative
O atom. |
Step 3:
An acid-base reaction. The alkoxide deprotonates a water molecule
reforming the catalyst hydroxide and the enol |
|
|
| |
|
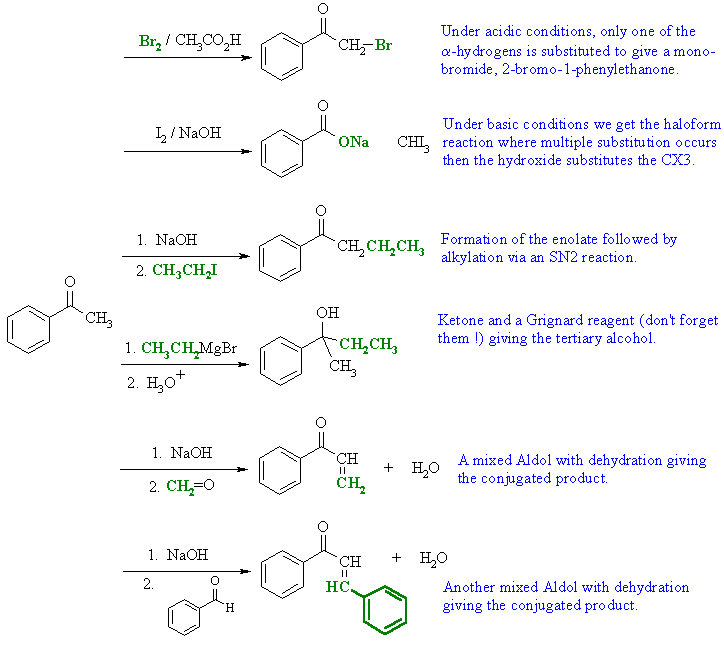
| Qu 4: |
Acetophenone
is a typical ketone..... |
| |
|
|
|
| Qu 5: |
As we have an
α,β-unsaturated ketone, then there could be
both direct (1,2-) or conjugate (1,4-) addition reactions: |
|
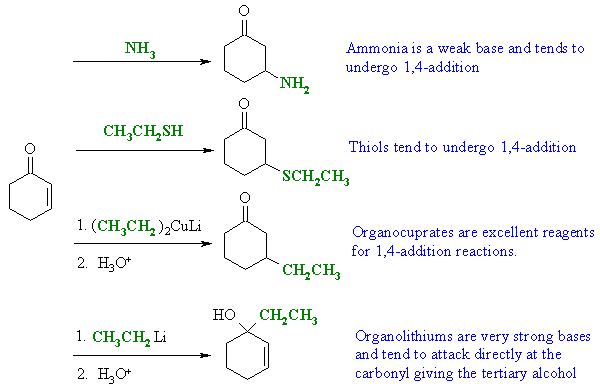
|
|
|