| Qu1: |
Increasing the
number alkyl substituents decreases the electrophilicity of the carbonyl
C due to: |
|
(i) electronic
effects (alkyl groups are weak electron donors) and (ii) steric effects
(inhibit Nu approach)
H2C=O >
CH3CHO > (CH3)2C=O
|
| Qu 2: |
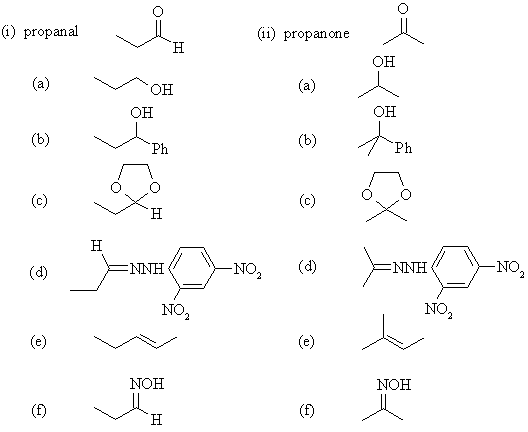
Note the very
similar nature of the reactions that the aldehyde and ketone undergo: |
|
|
|
| (a) LiAlH4
is a hydride reducing agent converting C=O to H-C-OH. |
| (b) PhMgBr is a Grignard
reagent. The Ph adds to the C=O to give Ph-C-OH. |
| (c) Alcohols react to
give acetals, in this case since it is a diol, we get cyclic acetals. |
| (d) The arylhydrazine
reacts to give the arylhydrazone via addition-elimination. |
| (e) The ylid undergoes
a Wittig reaction generating the alkene. |
| (f) Hydroxylamine
reacts to give the oxime via addition-elimination. |
|
| Qu 3: |
First step is
to recognise what the products are as that defines the nucleophilic atom. |
|
(i) Hydroxyl amine,
NH2OH , reacts through the N to give an oxime,
R2C=N-OH. N is more nucleophilic (better electron donor)
than O because the higher electronegativity of O makes it more difficult
to donate its' electrons.
(ii) Semicarbazide, NH2NHCONH2
reacts to give a semicarbazone. This reaction occurs through the
terminal amine type N rather than either of the amide type N
which are less nucleophilic due to the involvement of the electrons
in resonance.
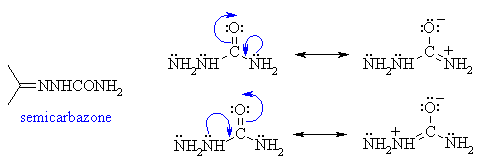 |
|
|
| Qu 4: |
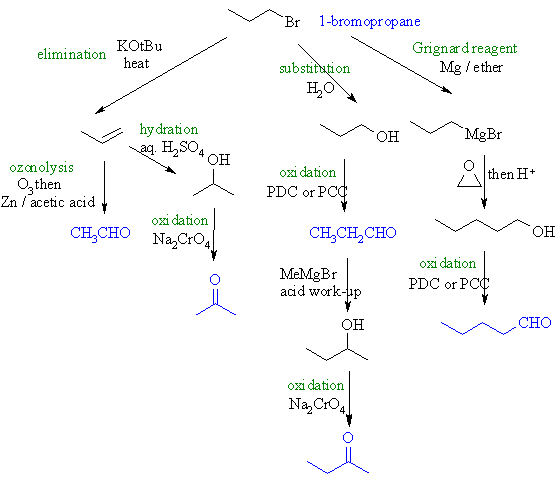
Here is a scheme
collecting possible syntheses together (based on the more important reactions) |
|
|
|
| (a) Ethanal
: we need to loose a C... ozonolysis of an alkene formed by elimination
of the alkyl halide would allow that and give the aldehyde. |
(b) Propanone
: oxidation of the secondary alcohol to get the ketone requires
that we eliminate then hydrate the alkene in Markovnikov fashion.
(c) Propanal : aldehydes can be obtained by oxidation
with the Cr reagents PDC (pyridinium dichromate) or PCC (pyridinium
chlorochromate) in methylene chloride (aq. conditions result in
over oxidation to the carboxylic acid). The primary alcohol is obtained
by substitution. |
| (d) 2-Butanone
: now we need to gain a C. We can do this with Grignard chemistry
by adding MeMgBr to the previous product, propanal, followed by
oxidation of the secondary alcohol. |
| (e) Pentanal
: this time we must add 2 C. Again use a Grignard, but this time
add ethylene oxide to the Grignard of the original halide. Finally,
controlled oxidation (see (c)). |
|
|
| Qu 5: |
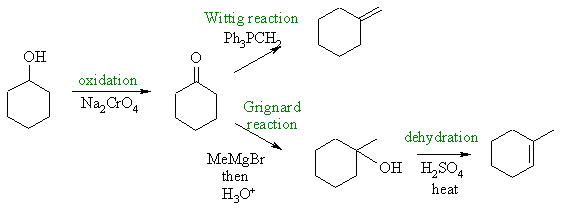
In both cases
we need to add a C atom so we need nucleophilic C systems which
react with cyclohexanone. |
|
Addition of a
Grignard reagent gives the tertiary alcohol, but this can only be eliminated
to give the more highly substituted endocyclic alkene. The Wittig
reaction is ideal for creating the exocyclic alkene since the WIttig reaction
specifically transforms a C=O to C=C at the same location.
|
|
|
