| Chapter 17: Aldehydes and Ketones. Nucleophilic Additionto C=O |
| Chapter 17: Aldehydes and Ketones. Nucleophilic Additionto C=O |
Friedel-Crafts Acylation
of Benzene
(review of Chapter 12)

Reaction type: Electrophilic Aromatic Substitution
Summary.

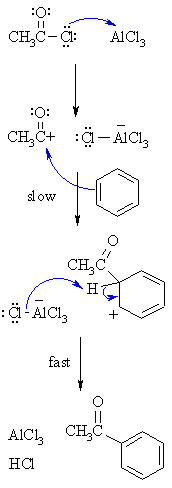
| MECHANISM FOR THE FRIEDEL-CRAFTS ACYLATION OF BENZENE | |
| Step 1:
The acyl halide reacts with the Lewis acid to form a a more electrophilicC, an acylium ion |
|
| Step 2:
The π electrons of the aromatic C=Cact as a nucleophile, attacking the electrophilic C+. This step destroysthe aromaticity giving the cyclohexadienyl cation intermediate. |
|
| Step 3:
Removal of the proton from the sp3 C bearing the acyl- groupreforms the C=C and the aromatic system, generating HCl and regeneratingthe active catalyst. |
|
| © Dr. Ian Hunt, Department of Chemistry |