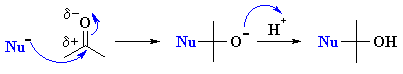|
Chapter 14: Organometallic
Compounds |
 |
Reactivity of Organometallics
As we have seen previously, the carbon
attached to the metal is anionic in character, so it reacts as a carbanion,
a nucleophilic carbon.
In principle there are 3 important
groups of reactions where nucleophiles attack
electrophilic C atoms.
For the organometallic reagents these types of reactions will result in the
formation of new C-C bonds.
Limitations will be discussed below.
| General Mechanism
|
Organometallic Application
|
| 1. Nucleophilic Substitution
Chapter 8 |
|

|
R2CuLi with
alkyl halides or tosylates to give alkanes |
|
|
| 2. Nucleophilic Addition
Chapter 17 |
|
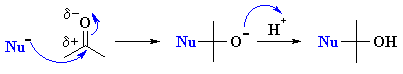
|
RLi or RMgX
with aldehydes or ketones to give 2o or 3o alcohols |
|
|
| 3. Nucleophilic Acyl
Substitution Chapter 20 |
|
 |
RLi or RMgX
with esters to give 3o alcohols |
Study Tip:
Note that the electrophilic C atoms can be recognised by realising that
they are connected to electronegative atoms (esp. halides or oxygen). |
Limitations:
- Organolithium, RLi, and
organomagnesium, RMgX, reagents are typically too basic to be used
in nucleophilic substitution reactions (1) with alkyl halides
or tosylates where they tend to cause elimination
reactions or other side reactions.
- Organocuprates, R2CuLi,
reagents are less reactive and do not react with aldehydes, ketones or esters
but can be reacted with alkyl halides or tosylates to give alkanes without
elimination.
- Nucleophilic acyl substitution
(3) reactions of organolithium, RLi, and organomagnesium, RMgX,
reagents are most commonly seen with esters.