| Qu 1 |
Use the following list of compounds to answer the questions
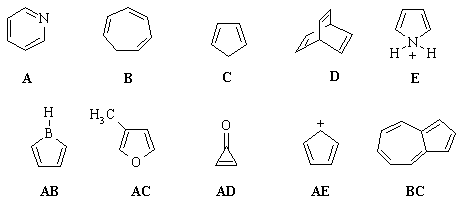
below: |
|
|
|
Select the compound that is best described as: |
|
(a) A neutral, 4 p-electron, anti-aromatic system. |
|
(b) A 6 p-electron, aromatic
system. |
|
(c) An aromatic system because n=2 in the Huckel 4n+2
rule. |
|
(d) A non-aromatic, conjugated 6 p-electron
system |
|
(e) A non conjugated hydrocarbon. |
|
(f) Non-aromatic as drawn, but if H- were removed would
give an aromatic cation. |
|
(g) Non-aromatic as drawn, but has an important resonance
structure that is aromatic. |
|
(h) Non-aromatic as drawn but has an aromatic conjugate
base. |
|
|
| Qu 2: |
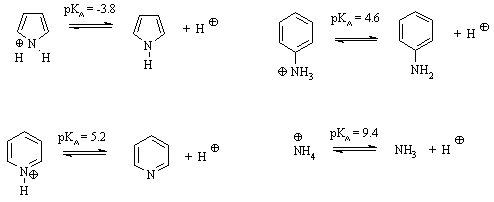
Consider the pKa data for the following
aromatic N containing systems: |
|
 Henderson-Hasselbach equation:
pKa = pH + log ([A-]/[HA])
Henderson-Hasselbach equation:
pKa = pH + log ([A-]/[HA]) |
|
Based on the scheme provided, |
|
(a) Which species is the strongest acid ? |
|
(b) At pH=4.2, what is the relative ratio of pyridine
to its conjugate acid ? |
|
(c) Which is the better base: pyridine or aniline,
and why ? |
|
(d) Why is pyrrole is a weaker base than pyridine? |
|
|
| Qu 3: |
Rate the resonance energies (in comparison to one another)
of each of the following: |
|
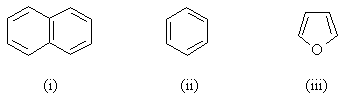
|
|
|
| Qu 4: |
Rate the relative stability of the following carbocations
(in comparison to one another): |
| |
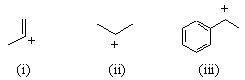
|
|
|
| Qu 5: |
Use the following data to answer the questions below: |
|
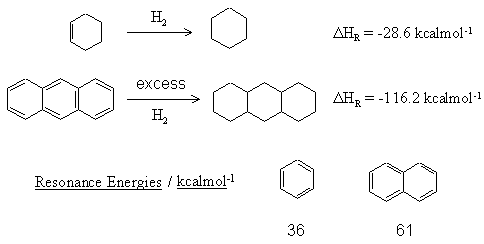
|
|
(a) Calculate the resonance energy of anthracene, 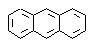 |
|
(b) Anthracene is known to react with maleic anhydride
in a Diels-Alder reaction. Predict which of the products shown is
obtained. Why ?
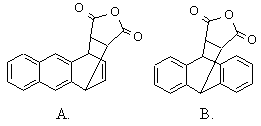
|