| Chapter 11: Arenes and Aromaticity |
| Chapter 11: Arenes and Aromaticity |
Reaction of Alkyl Benzenes with Halogens
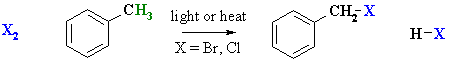
Reaction type: Radical Substitution
Summary
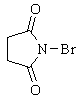 |
N-Bromosuccinimide |
Related reactions
|
|
|
| Step 1 (Initiation) Heat or uv light cause the weak halogen bond to undergo homolytic cleavage to generate two bromine radicals and starting the chain process. |
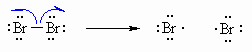
|
| Step 2 (Propagation) (a) A bromine radical abstracts a hydrogen to form HBr and a benzyl radical, then (b) The benzyl radical abstracts a bromine atom from another molecule of Br2 to form the benzyl bromide product and another bromine radical, which can then itself undergo reaction 2(a) creating a cycle that can repeat. |
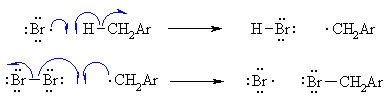
|
| Step 3 (Termination) Various reactions between the possible pairs of radicals allow for the formation of Br2 or the product, benzyl bromide. These reactions remove radicals and do not perpetuate the cycle. |
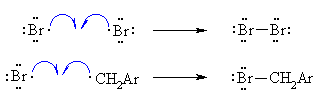
|
| © Dr. Ian Hunt, Department of Chemistry |