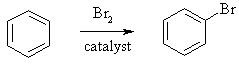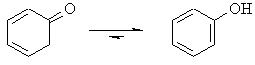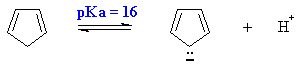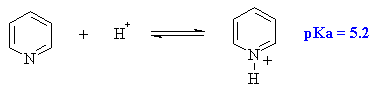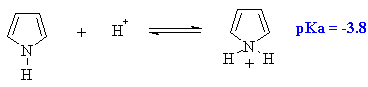 |
Aromatics tend to undergo substitution and retain the aromatic
unit rather than lose it as would be the case if electrophilic addition
occurred as is the case with alkenes. |
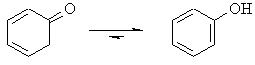 |
The ketone form of the tautomerisation is usually preferred.
Not so here where the stability of the aromatic product makes the
enol of the phenol form more favourable. |
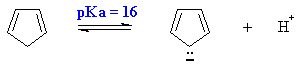 |
Hydrocarbons are not usually very acidic (pKa > 50). But cyclopentadiene
has a much lower pKa due to the aromatic stability of its aromatic conjugate
base.
What other systems have a pKa = 16 ? ANSWER
|
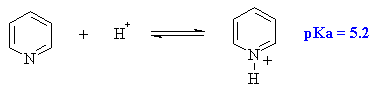 |
Pyridine is like benzene but an N has replaced one CH. The N atom is
weakly basic since the lone pair is in an sp2 hybrid orbital.
Is the conjugate acid aromatic ? ANSWER |
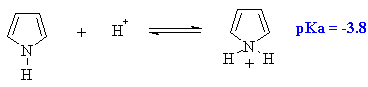 |
Pyrrole is a much weaker base than pyridine (see above). This is because
the lone pair on the N atom is already involved in the aromatic array
of p electrons. Protonation results in loss of aromaticity and is therefore
unfavourable. |