|
Structure and Preparation of Alkenes. Elimination Reactions |
|
Structure and Preparation of Alkenes. Elimination Reactions |
| Qu 1: | When heated with H2SO4, 2o alcohols undergo dehydration via an E1 mechanism. |
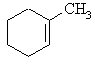 The major product
is the tri-substituted alkene, methylcyclohexene The major product
is the tri-substituted alkene, methylcyclohexene
|
|
| Qu 2: | When heated with strong bases such as NaOEt, alkyl bromides undergo E2 elimination. |
| The outcome of E2 reactions is dependent on the antiperiplanar arrangement of the C-H and C-LG bonds. For substituted cyclohexanes this requires that the LG be axial. | |
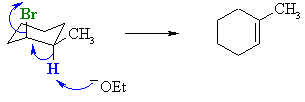 For the cis-isomer with the -Br axial, the more highly susbtituted alkene can be formed by removal of the H adjacent to the methyl group. |
|
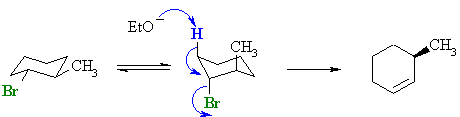 For the
trans-isomer, when the -Br is
axial the methyl group is also axial. Therefore the elimination must
occur from the C3-H bond giving the anti-Zaitsev product. For the
trans-isomer, when the -Br is
axial the methyl group is also axial. Therefore the elimination must
occur from the C3-H bond giving the anti-Zaitsev product. The reactive conformation is an unfavourable diaxial conformer, therefore the reaction will be slower than that of the cis-isomer. |
|
| Qu 3: | These are elimination reactions : | ||||
(a) First the E2 reaction of an alkyl halide
with a strong base. There two possible H atoms at C3 that can be removed
to give 2-butene, look at each in turn:
|
|||||
(b) Now the E1 reaction of an alcohol with
a strong acid. Again there are two possible H atoms at C3 to consider:
Implications: The steric interactions in the product forming steps control the stereoselectivity favouring trans-2-butene. |
| Qu 4: | The lowest energy conformation of menthyl chloride has the chlorine atom in a equatorial postion. |
| In this position there is no antiperiplanar H , ring
flip is difficult as it would require to formation of a triaxial conformer.
In contrast, in neomenthyl chloride, the lowest energy confromation has the chlorine atom axial with 2 H in the correct orientation to give the products. The major product is the more highly substituted alkene.
|
| © Dr. Ian Hunt, Department of Chemistry |