|
Structure and Preparation of Alkenes. Elimination Reactions |
|
Structure and Preparation of Alkenes. Elimination Reactions |
E2 Stereochemistry
E2 reactions occur most rapidly when the H-C bond and C-LG bonds involved are co-planar, most often at 180o with respect to each other. This is described as an antiperiplanar conformation. This conformation positions the σ bonds that are being broken in the correct alignment to become the π bond.
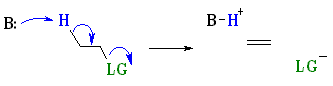
|
||

|
The staggered, antiperiplanar alignment is preferred because it aligns the two σbonds that become the π bond. | 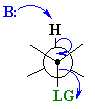 |
|
|
The JMOL image to the left allows you to highlight the H that is antiperiplanar to the Br atom and look at this from different view points. Try making it look like the Newman projection on the right above. | |
Synperiplanar arrangments where the angle between
the H-C bond and C-LG
is 0o are also known, usually in systems that are either inflexible
rings or intramolecular eliminations.
 |
The eclipsed, synperiplanar alignment also aligns the two σ bonds that become the π bond, but is less favourable than the antiperiplanar arrangement. | 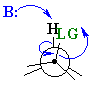 |
These alignments are example of a stereoelectronic effect because they involve the specific spatial postioning of the bonds (electrons) in order for the process to occur.
Implications:
trans-
cis- |
 |
The cis- isomer undergoes elimination
over 500 times faster than the trans- isomer.
In the cyclic system, in order for the preferred antiperiplanar arrangement favoured by E2 reactions, the C-H and C-LG bonds both need to be axial. |
Recall that in chapter 3 that we learnt that the t-butyl group has a strong preference for the equatorial position on cyclohexanes and
acts as a "lock". In the trans isomer, this means that the -Br is also equatorial and is therefore anti to C-C
bonds, not C-H. Since the cyclohexane is locked it cannot ring
flip into the geometry required for the E2 and elimination is slow.
In contrast, in the cis isomer, the -Br
is axial and is anti to 2 C-H bonds and the E2 occurs rapidly. Use the JMOL images
below to show the antiperiplanar bonds if you need to.
|
|
| © Dr. Ian Hunt, Department of Chemistry |