| Chapter 4: Alcohols and Alkyl Halides |
| Chapter 4: Alcohols and Alkyl Halides |
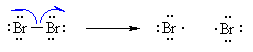
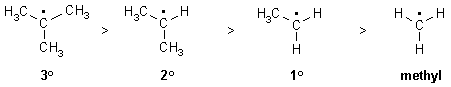
This is because alkyl groups are weakly electron donating
due to hyperconjugation and inductive effects.
Note that this is the same order as for carbocations. Resonance
effects can further stabilise radicals when present.
| Alkyl radicals are sp2 hybridised, planar
systems at the radical C centre. The p-orbital that is not utilised in the hybrids contains the single electron. |
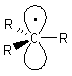 |
Reactivity:
As they have an incomplete octet, radicals are excellent electrophiles and react
readily with nucleophiles.
Alternatively, loss of H. can generate
a π bond
Rearrangements:
Unlike carbocations, radicals do not tend to undergo rearrangements
| © Dr. Ian Hunt, Department of Chemistry |