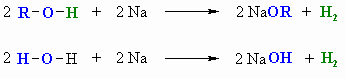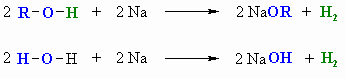 |
Chapter 4: Alcohols and Alkyl Halides |
 |
Alcohols
Nomenclature:
Functional group suffix = -ol
Functional group prefix = hydroxy-
Review alcohol
nomenclature ?
Primary, secondary or tertiary ? Alcohols are described
as being primary (1o), secondary (2o) or tertiary (3o)
depending on how many alkyl substiutents are attached to the carbon that carries the -OH unit.
|
|
|
|
ethanol
|
2-propanol
|
t-butanol
2-methyl-2-propanol
|
|
1o
|
2o
|
3o
|
Check the designations of primary, secondary and tertiary by
counting the number of C atoms attached to the C with the -OH attached.
Physical Properties:
- The polar nature of the O-H bond (due to the electonegativity
difference of the atoms ) results in the formation of hydrogen bonds
with other alcohol molecules or other H-bonding systems (e.g. water).
The implications of this are:
- high melting and boiling points compared to analogous
alkanes
- high solubility in aqueous media
Structure:
- The alcohol functional group consists of an O atom bonded
to a C atom and a H atom via σ bonds.
- Both the C-O and the O-H bonds are polar due to the high
electronegativity of the O atom.
Reactivity:
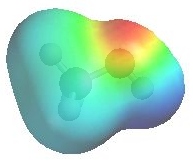 |
The image shows the electrostatic potential for methanol.
The more red an area is, the higher
the electron density and the more blue
an area is, the lower the electron density.
- The alcohol O atom are a region of high electron
density (red) due to the lone
pairs.
(red)
- Alcohol oxygen atoms are Lewis bases.
- So alcohols can react as either bases or nucleophiles
at the oxygen.
- There is low electron density (blue)
on H atom of the -OH group alcohol, i.e. H+ character.
- So alcohols are acidic (pKa ~ 16).
- Removal of the proton generates the alkoxide.
- The -OH group is a poor leaving group and needs
to be converted to a better leaving group before substitution can
occur.
|
Acidity:
- Due to the electronegativity of the O atoms, alcohols
are slightly acidic (pKa 16-18)
- The anion dervived by the deprotonation of an alcohol
is the alkoxide.
- Alkoxides are important bases in organic chemistry.
- Alcohols react with Na (or K) like water to give the
alkoxide: