| Chapter 2 : Alkanes |
| Chapter 2 : Alkanes |
| Ans 1:
|
No, a π bond is not symmetric with respect to the internuclear axis. Rotation about the axis along the bond between the two nuclei produces no change in the view of a σ bond (top pair) but rotation about the axis along the bond switches the lobes of the orbitals in the π bond (bottom). | 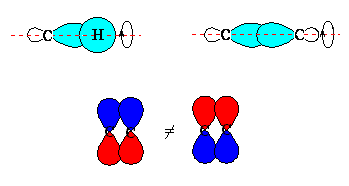 |
Ans 2 : Allene H2C=C=CH2 first look at how many groups each C is attached to, the answer is colour coded below:
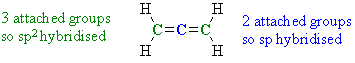
Therefore, there are 2 sp2 and 1 sp hybridised C atoms. Note that this example highlights an example of an sp hybridised C that is not part of an alkyne C≡CAns 3 : Again we need to look at the groups attached to the C atoms in question:
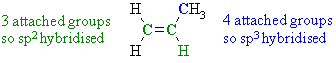
Therefore the bond that attaches the methyl group to the double bond is a σ bond formed by the intereraction of a C sp3 and a Csp2 orbitals.
| Ans 4:
|
The molecule H22-
is isoelectronic with He2,
so like diatomic helium, this is an unstable molecule. In order to be able to "work-out" an answer to the problem, we should draw out the molecular orbital diagram for the interaction of two H atoms as shown to the right. The only difference compared to regular H2 is the number of electrons. Since H22- is a four electron system, we are forced to use the s*-anti-bonding orbital so destabilising the system. |
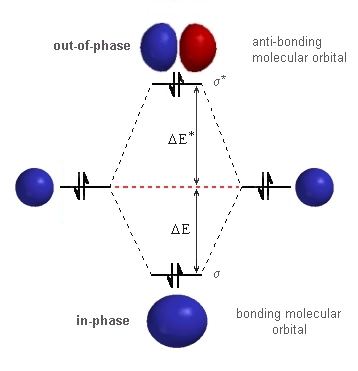 |
| © Dr. Ian Hunt, Department of Chemistry |