| Chapter 2 : Alkanes |
| Chapter 2 : Alkanes |
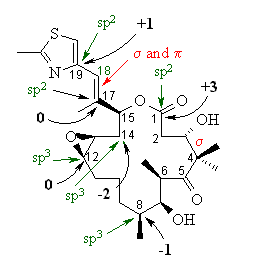
| Most of the answers are shown on the structure
of EPOTHILONE A to the right.
(a) The hybridisation of the C atoms asked are indicated in green. If you got them wrong, it may be because you forgot to account for the CH bonds that aren't shown. (b) C14-C15 > C15-C17 > C17-C18. The hybridisation of the C atoms involved in the various bonds affects the bonds lengths. The greater the % s character in the hybrids (sp3 = 25% s, 75% p : sp2 = 33% s, 67 % p : sp = 50% s, 50% p), the shorter the bond will be. C14-C15 is sp3-sp3, C15-C17 is sp3-sp2 and C17-C18 is sp2-sp2. (c) The bonding MOLECULAR orbitals are indicated in red. (d) The oxidation states are indicated in black. If you got them wrong, it may be because you forgot to account for the CH bonds that aren't shown. (e) There are 5 C-C σ bonds where at least one C is not sp3 hybridised : |
 |
| © Dr. Ian Hunt, Department of Chemistry, University of Calgary |