 |
Chapter 2 :
Alkanes |
 |
Oxidation
and Reduction
- An important part of the
task of electron book-keeping.
- Oxidation, [O], and
reduction, [R], are opposites and both must occur
simultaneously,
hence redox reactions.
- Organic chemists will
normally describe a reaction as either oxidation
or reduction depending on the fate of the major organic component.
Oxidation:
- more C-O bonds
(or other atoms more electronegative than C)
- less C-H bonds
- loss of electrons
- increased oxidation
state, e.g. +1 to +3 (see below)
Reduction:
- more C-H bonds
- less C-O bonds
(or other atoms more electronegative than C)
- gain of electrons
- decreased oxidation
state, e.g. +1 to -1 (see below)
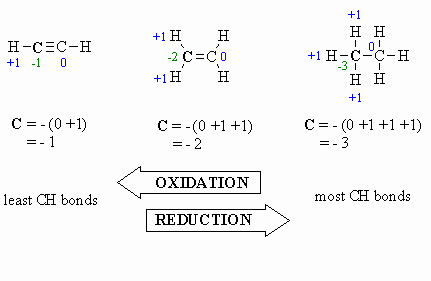
Calculating Oxidation Number
or State (there are several methods for doing this, pick the one that works
for you !)
This allows for a more formal, quantitative decription of the oxidation state
and is based on looking at the relative electronegativity of the atoms attached
to the atom being assessed. The algebraic sum of the oxidation states must equal
the charge of the molecule.
If we are looking at a central carbon
atom:
- for attached C atoms, i.e. C-C bonds electrons
shared, count 0
- for attached X atoms, i.e. C-X bonds (X
more electronegative), count -1 (per bond)
- for attached H atoms, i.e. C-H bonds (H
is less electronegative than C), count +1
- Add the total for atoms attached to the C in question,
then switch the sign.
If we are looking at a generic central atom
- for attached atom with the same electronegativity, i.e.
bonding electrons shared, count 0
- for attached X atoms, i.e. X more electronegative,
count -1 (per bond)
- for attached Y atoms, i.e. Y is less electronegative,
count +1(per bond)
- Add the total for atoms attached to the central atom
question, then switch the sign.
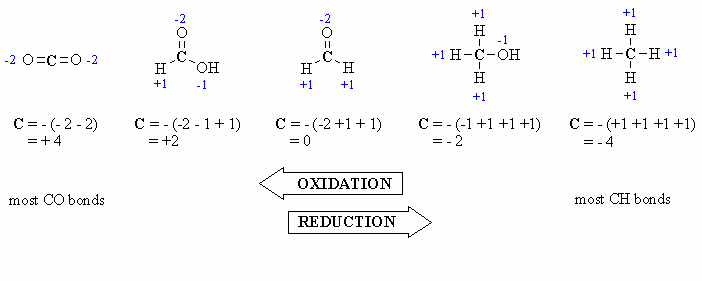
Here are a few examples, and a couple of
schemes that show important relationships:

Study Tip:
In the past you may have learnt that O is always -2, however it is
better to count each bond to an O as -1 since there is a difference between
C=O and C-O as seen by comparing the aldehyde and the alcohol
in the diagram above. After all , the -2 statement is wrong.... e.g.
in oxygen gas, O2, the oxidation state is zero |
 |
© Dr. Ian Hunt,
Department of Chemistry, University of Calgary |
 |




