Chapter 1 : Answers to bond types questions
 |
Chapter 1: Structure Determines Bonding |
 |
Answers to Questions relating to Bonds and Bond Polarity
1. What types of bond exists between the atoms in a molecule of
methyl lithium, CH3Li ?
The formula for this compound can be
written as [CH3-][Li+] because the metal
is very electropositive compared to the carbon atom (if we
look at their electronegativities, C = 2.5, Li = 1.0). The [CH3-]
group, a methyl anion, has covalent bonds between carbon and hydrogen.
Though there is no significant bond polarity to a C-H bond there is a molecular
polarity to the anion because of the negative charge placed on carbon.
The attraction of the Li+ cation to CH3-
is essentially ionic.
2. What types of bonds exist between the atoms in a molecule of sodium
sulfate, Na2SO4
?
The formula for this compound can be
written as [Na+]2[SO42-], the
sulfate anion is formed by covalent bonding between sulfur and oxygen.
Since there is a bond polarity to a S-O bond these are polar covalent bonds.
The attraction of the Na+ cations to SO42-
is ionic.
3. What types of bonds exist between the atoms in a molecule of chlorine
gas, Cl2 ?
A non-polar covalent bond exists
between the atoms in a molecule of Cl2.
4. What are all the bond polarities in
a sodium acetate molecule, CH3COONa ?
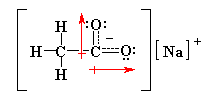 |
The structure is shown as the resonance
hybrid (so both O atoms have equal partial -ve charges).
The most significant polar bonds are the
CO bonds.
Since H and C have very similar electronegativities
(2.2 and 2.5 respectively), the CH bonds are essentially non-polar. |