| Chapter 1: Where are the electrons ? |
| Chapter 1: Where are the electrons ? |
Orbital
Filling memory aid
1. Write a list of the orbitals, starting a new line for each new principal quantum number (n):

2. Draw diagonal arrows
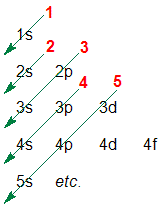
3. Follow the arrows, starting at the top and read off the order of the orbitals....this means the orbital energies list from lowest (most stable) to highest (least stable) and hence the orbital filling order is 1s 2s 2p 3s 3p 4s etc.
 |
© Dr. Ian Hunt, Department of Chemistry |