Part 6: THERMODYNAMICS
This should have been a reasonably straight forward calculation, but care needs to be taken with the last part of the question.
a. C5H8 + 7 O2 --> 5 CO2 + 4 H2O (1.5 marks)
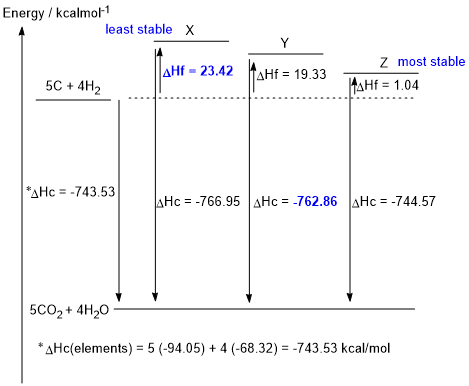
b. DHc elements = (5 x -94.05) + (4 x -68.32) = -743.53 kcal/mol
DHf X= -743.53 - (- 766.95) = 23.42 kcal/mol (1.5 marks)
DHc Y = -743.53 - (19.33) = -762.86 kcal/mol (1.5 marks)
c. (4 marks)

d. see above (
e.
 (1.5 marks)
(1.5 marks)
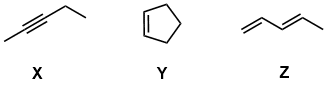
Given the thermodynamic data in the table provided in the question, and hence the figure shown above, X is the least stable isomer and Z is the most stable isomer (i.e. lowest energy)
Given the structures, we need to consider several factors. (1) C-C sigma bonds are stronger than pi bonds (by about 60 kcal / mol (2) sp2 C-H are stronger than sp3 C-H by about 10 kcal / mol and (3) resonance will further stabilise the conjugated diene (4) 2nd pi bond in an alkyne is weaker than the pi bond in an alkene and (5) branching and hence primary C-H bonds > secondary C-H > tertiary C-H in terms of strength but only by about 2 kcal per change (hence they aren't significant in this case to be relevant).
Z is the most stable due to higher number of stronger sp2 C-H bonds plus the resonance interaction of the two C=C. The cycloalkene has traded a pi bond for an addtional stronger C-C sigma bond compared to a weaker pi bond.
f. Weaker C-H due to being secondary and allylic to 2 C=C:

Common errors: