 |
|
 |
|
|
Misconception Alert!
It is often INCORRECTLY taught in High School that it is the lowest sum of locants that determines the numbering scheme. |
See here for more discussion on the first point of difference rather than summing locants (don't sum!) follow this link.
STUDY TIP
|
|
Here are some illustrative examples of the first point of difference and related rules:
| 2-methylpentane not 4-methylpentane. |
 |
Here the methyl group is given the lowest number by numbering
from the right (2- rather than 4-). |
| 2,2,4-trimethylpentane not 2,4,4-trimethylpentane |
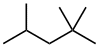 |
The first difference is at the second locant - by numbering from the right, the second locant is kept lower (2- rather than 4-). |
| 2,4,4-trimethylhexane not 3,3,5-trimethylhexane |
 |
The first difference is at the first locant - by numbering from the left, the first locant is kept lower (2- rather than 3-). |
| 1-ethyl-2-methylcyclohexane not 2-ethyl-1-methylcyclohexane or 1-ethyl-6-methylcyclohexane |
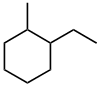 |
The first difference is at the second locant (2- rather than 6-) which means 1,2- is preferred over 1,6-. The assignment of these locants is then dictated by the alphabetisation : ethyl preceeds methyl so ethyl gets the lower number. |
| pentan-2-ol not pentan-4-ol |
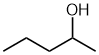 |
Here
the principal functional group, the alcohol -OH is give the lowest
locant by numbering from the right (2- rather than 4-). |
| pentan-3-ol no choice ! |
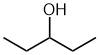 |
No
matter which way this is numbered, the -OH is at C3. |
| 4-methylpentan-2-ol not 2-methylpentan-4-ol |
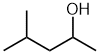 |
The principal functional group (-OH) gets the lowest locant - by numbering from the right (2- rather than 4-). |
| 2-methylpentan-3-ol not 4-methylpentan-3-ol |
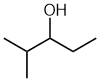 |
The first difference is in the methyl locant - since the -OH must be at C3 either way, but by numbering from the left, the methyl locant kept lower (2- rather than 4-). |
| ©Dr. Ian Hunt, Department of Chemistry |