 |
Useful Concepts |
 |
Thermodynamics and
Stability (for the organic chemist)
- The lower the potential energy of the system, the more stable it is.
- Chemical processes usually occur because they are thermodynamically
favourable.
- "Thermodynamically favourable" means from high energy to low
energy,
or, put another way, from less stable to more stable.
- Understanding the relative stability of molecules can be important for
predicting relative reactivity of starting materials and the relative
yields
of potential products.
- Stability can be determined by comparing experimentally measured or
based
on theoretical calculations.
- It is important when determining relative stabilty to compare isomeric
systems.
Heat of
Reaction ΔHro
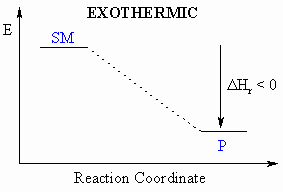
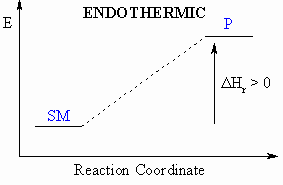
- Defined to be the heat released during a particular reaction.
- If heat is released during this process, then the
reaction
is exothermic ( = heat given out)
- If heat is absorbed during this process, then the
reaction
is endothermic ( = heat taken in)
However, there are certain types of
reactions that are particularly
common for making thermodynamic comparisons:
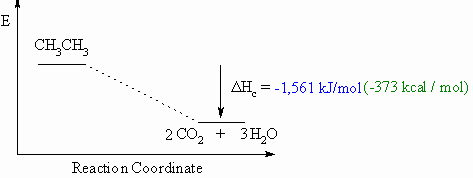
Heat of Combustion, ΔHco
- Defined to be the heat released when one mole of a compound undergoes
complete
combustion in O2.
- This will usually be an exothermic process, as shown in the
example
below.
- When drawing these diagrams, it is important to make sure they are balanced.
- Note that for heats of combustion, the organic compound is a starting
material for the reaction.
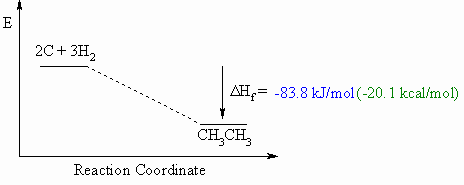
 Heat of Formation, ΔHf o
Heat of Formation, ΔHf o
- Defined to be the heat released if one mole of a compound were formed
from
its component elements in their standard state.
- These diagrams can be either endothermic or exothermic processes.
- When drawing these diagrams, it is important to make sure they are balanced.
- Note that for heats of formation, the organic compound is a product for the reaction.
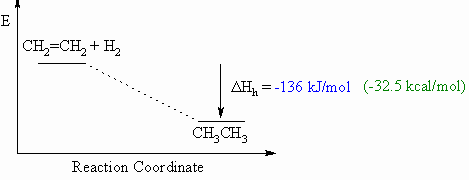
 Heat of hydrogenation, ΔHho
Heat of hydrogenation, ΔHho
- Defined to be the heat released upon the addition of H2 to
one
mole of a compound (e.g. an alkene or alkyne) to generate the
corresponding
alkane.
- This will usually be an exothermic process, as shown in the
example
below for ethene to ethane, since 2 σ bonds
are made from each π bond and each H-H σ
bond.
- When drawing these diagrams, it is important to make sure they are balanced (note the inclusion of both starting materials).
- Note that for heats of hydrogenation, both starting materials and products are organic compounds.
Hess's Law
Although not specifically covered in most
organic text books, Hess's
Law is very useful when investigating the thermodynamics of
reactions.
Hess's Law can be expressed by the following expression and diagram:
"The enthalpy change for a reaction (ΔHro)
that converts starting materials to products is independent of the
reaction
pathway"
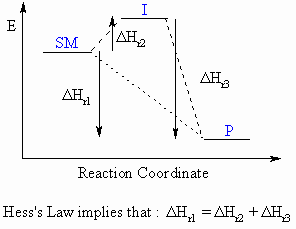 |
For the reaction SM
to P which can either
proceed directly or via an intermediate, I, the overall enthalpy change
for the converssion of SM to P must be the same.
i.e. ΔHSM-Po = ΔHSM-Io + ΔHI-Po
|
Essentially, it can be treated as the
addition of 2-dimensional vectors,
and CARE is required with the signs of the ΔHo terms.