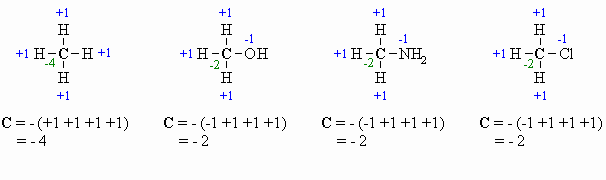 |
Useful Concepts |
 |
Oxidation and Reduction
- An important part of the
task of electron book-keeping.
- Oxidation, [O], and
reduction, [R], are opposites and both must occur
simultaneously,
hence redox reactions.
- Organic chemists will
normally describe a reaction as either oxidation
or reduction depending on the fate of the major organic component.
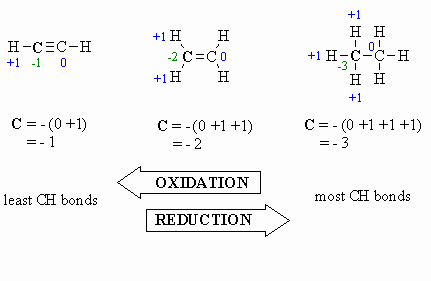
Oxidation:
- more C-O bonds (or other
atoms more electronegative than C)
- less C-H bonds
- loss of electrons
- increased oxidation
state, e.g. +1 to +3 (see below)
Reduction:
- more C-H bonds
- less C-O bonds (or other
atoms more electronegative than C)
- gain of electrons
- decreased oxidation
state, e.g. +1 to -1 (see below)
Calculating
Oxidation Number or State (there
are several methods for doing this, pick the one that works for you !)
This allows for a more formal, quantitative decription of the oxidation
state for the C atoms and is based on looking at what atoms are
attached
to the C atom in question. The algebraic sum of the oxidation states
must
equal the charge of the molecule.
- for attached C atoms, i.e. C-C bonds electrons shared, \count
0
- for attached X atoms, i.e. C-X bonds (X more electronegative), \count
-1 (per bond)
- for attached H atoms, i.e. C-H bonds (H is less electronegative
than C), \ count +1
- Add the total for atoms
attached to the C in question, then switch the
sign.
Here are a few examples, and a couple of
schemes that show important relationships:
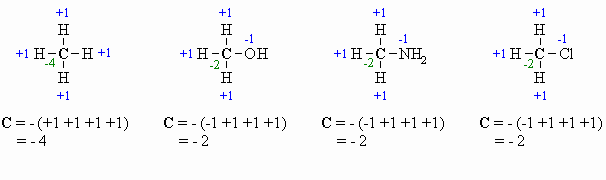
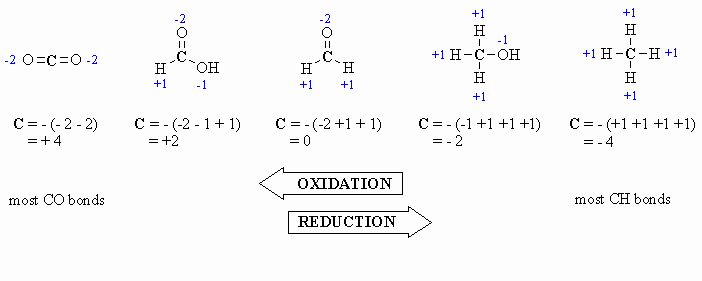
Tips: In General Chemistry or
High School you may have
learnt that O is always -2, however it is better to count each
bond
to an O as -1 since there is a difference between C=O and C-O as seen
by
comparing the aldehyde and the alcohol in the diagram above. After all
, the -2 statement is wrong.... for example in oxygen gas, the
oxidation
state is zero