| Useful Concepts |
| Useful Concepts |
Nuclear Magnetic Resonance (NMR) Spectroscopy
NMR occurs due to the absorbance of radio
frequency radiation to cause
the "flipping" of nuclear spins from low to high energy spin states.
While not all nuclei are NMR active (e.g. 12C
and 16O are inactive), the most important nuclei for organic
chemists are 1H and 13C (both with nuclear spin
=
1/2).
1H (or proton) is the most common, and the one we
will
spend most time talking about.
There are 4 levels of information to be gained from proton NMR related to different aspects of the spectrum:
1. How many types of H ? ..........Look at
how many basic groups of
signals there are.
2. How many H of each type ? ....Look at the integration (relative area) of each group.
3. What is each type ? .................Look at the chemical
shiftof each group and relate to tables of typical values.
4. What is the connectivity ? ........Look at the coupling
patterns. This tells you what is next to each group
Integration = Measure of the area of the peaks which is proportional to the number of H that the peak represents.
Chemical shift = Position on the scale (in ppm) where the peak occurs. For ranges of typical values, see the figure below. There are two major factors that cause different chemical shifts (a) deshielding due to reduced electron density (due electronegative atoms) and (b) anisotropy (due to π bonds).
Coupling = Due
to the proximity of "n"
other equivalent H atoms, causes the signals to be split into (n+1)
lines.
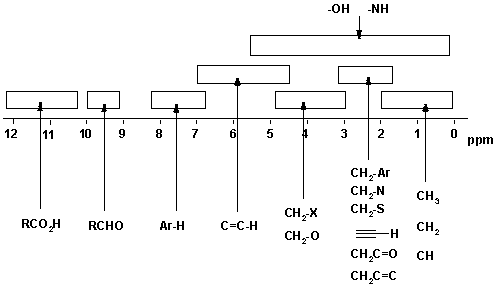
Deshielding: The electrons around the
proton create a magnetic field that opposes the applied field. This
reduces
the field experienced at the nucleus and therefore decreases the
freqency
required for the absorption. Therefore the chemical shift (delta /ppm)
will change depending on the electron density around the proton. Since
electronegative groups decrease the electron density, there will be
less
shielding (ie. deshielding) and the chemical shift will increase.
Anisotropy: This just means "a non-uniform" magnetic field. Electrons in pi systems (e.g. benzene, alkenes, alkynes, carbonyls etc.) interact with the applied field and are caused to "circulate" within the pi system. This motion induces a magnetic field that causes the anisotropy. As a result, the nearby protons will experience 3 fields: the applied field, the shielding field of the valence electrons and the field due to the pi system. Depending on the position of the proton in this third field, it can be either shielded or deshielded, which implies that the energy required for, and the frequency of the absorption will change.
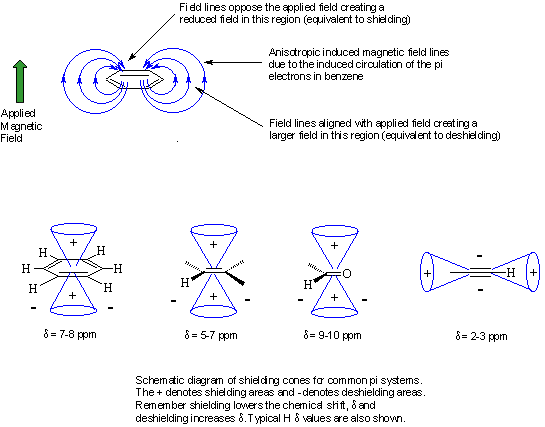
Be aware that the exact substitution pattern around a particular H causes changes in the chemical shift and therefore ranges of values are given in the tables and the above figure. You will normally be given copies of the tables in exams, but you will save yourself lots of time, and avoid confusing yourself, if you know approximately where the various types of H occur.
It is useful to compare and contrast H-NMR and C-NMR as there are certain differences and similarities:

| © Dr. Ian Hunt, Department of Chemistry |