| Useful Concepts |
| Useful Concepts |
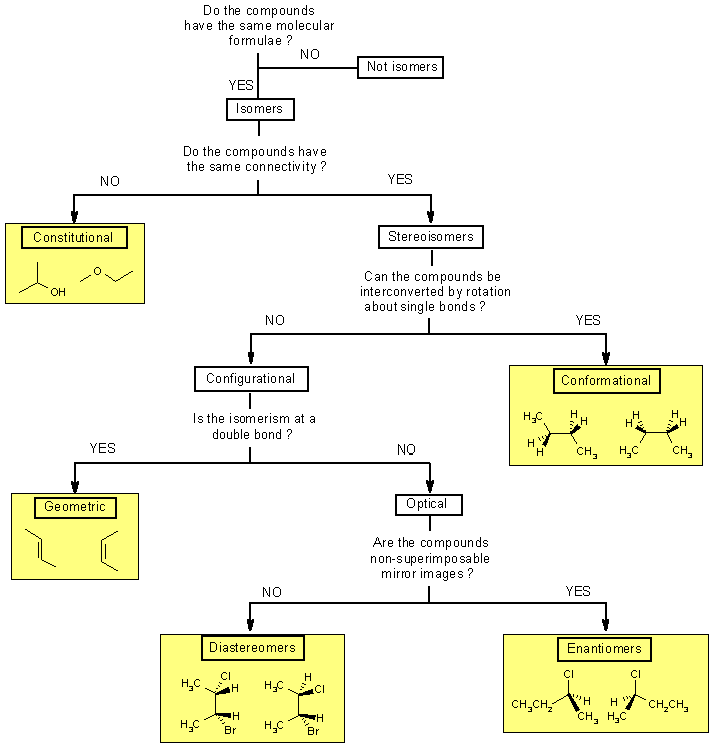
Compounds that have the same molecular formula but different chemical structures are called isomers. Remember isomerism is a property between a pair (or more) of molecules, i.e. a molecule is an isomer of another molecule. A similar relationship is that of brother or sister... you can only be a brother or sister to someone else.
Depending on the nature of the difference between the structures, it is possible to classify isomers into various sub-types. The following tree diagram should help you recognise the differences based on a simple YES / NO question. If you "click" on the named boxes there is a link to a definition and an example.
|
|||||
|
Isomers are compounds with the same molecular formulae but that are structurally different in some way. It is important to be able to recognise isomers because they can have different chemical, physical properties and biological properties.
|
|||||
|
Constitutional isomers (or structural isomers) differ in the order in which the atoms are connected so they can contain different functional groups and / or bonding patterns (e.g. branching)
|
|||||
|
Stereoisomers have the same functional groups and connectivities, they differ only in the arrangement of atoms and bonds in space.
|
|||||
|
Conformational isomers (or conformers or rotational isomers or rotamers) are stereoisomers produced by rotation about σ bonds, and are often rapidly interconverting at room temperature (review Chapter 3 ?)
|
|||||
|
Configurational isomers are stereoisomers that do not readily interconvert at room temperature and can (in principle at least) be separated. |
|||||
|
Geometric isomers are configurational isomers that differ in the spatial position around a bond with restricted rotation (e.g. a double bond):
|
|||||
|
Optical isomers are configurational isomers that differ in the 3D relationship of the substituents about one or more atoms. |
|||||
|
Enantiomers are optical isomers that are non-superimposable mirror images.
|
|||||
|
Diastereomers are stereoisomers that are not enantiomers.
|
|||||
| © Dr. Ian Hunt, Department of Chemistry |