| Useful Concepts |
| Useful Concepts |
Hyperconjugation is the stabilising interaction that results from the interaction of the electrons in a s-bond (usually C-H or C-C) with an adjacent empty (or partially filled) p-orbital or a p-orbital to give an extended molecular orbital that increases the stability of the system.
Based on the valence bond model of bonding, hyperconjugation can be described as "double bond - no bond resonance" but it is not what we would "normally" call resonance, though the similarity is shown below.
What is the key difference between
hyperconjugation and resonance ? ![]()
Hyperconjugation is a factor in explaining
why increasing the number
of alkyl substituents on a carbocation or radical centre leads to an
increase
in stability.
| Let's consider how a methyl group is involved in hyperconjugation with a carbocation centre. |
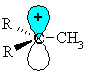 |
| First we
need to draw it to show the C-H s-bonds.
Note that the empty p orbital associated with the positive charge at the carbocation centre is in the same plane (i.e. coplanar) with one of the C-H s-bonds (shown in blue.) |
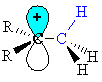 |
| This
geometry means the electrons in the s-bond
can be stabilised by an interaction with the empty p-orbital of the
carbocation
centre.
(this diagram shows the similarity with resonance and the structure on the right has the "double bond - no bond" character) |
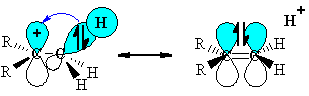 |
The stabilisation arises because the
orbital interaction leads to the
electrons being in a lower energy orbital
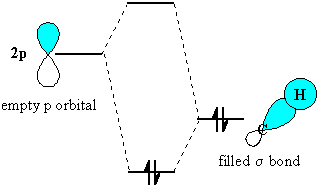
Of course, the C-C s-bond is free
to rotate, and as it does so, each of the C-H s-bonds
in turn undergoes the stabilising interaction.
So the ethyl cation has 3 C-H s-bonds
that can be involved in hyperconjugation.
The more hyperconjuagtion there is, the greater the stabilisation of
the system.
So for example, the t-butyl cation has 9 C-H s-bonds
that can be involved in hyperconjugation. Hence (CH3)3C+
is more stable than CH3CH2+
The effect is not limited to C-H s-bonds,
appropriate C-C s-bonds can also be
involved
in hyperconjugation.
| © Dr. Ian Hunt, Department of Chemistry |