 |
Useful Concepts |
 |
Aromaticity
Aromaticity is a property associated with
the extra stability of certain
types of π systems due to the nature of the
molecular orbitals. It is not restricted to substituted benzenes,
6-membered
rings or just hydrocarbons.
Based on the properties of compounds,
there are FOUR criteria about
the π system that need to be met in order for
the "special" aromatic stabilisation to be observed:
- Conjugated (there needs to one "p"
orbital from each atom in the
cycle, so each atom must be either sp2 or sp hybridised)
- Cyclic (Linear
systems are not aromatic)
- Planar (so
there is good overlap /
interaction between the "p" orbitals....
not always easy to consider)
- The Huckel Rule.....
4n+2 π electrons
( this is equivalent to an odd number of π -electrons
pairs) in the cyclic conjugated system.
Study Tip : One way to think about the
first 3 criteria is to compare
the p orbitals of the system to fence posts surrounding a field. The
posts
need to be regularly spaced, all around the field with all the posts
standing
upright in order to have an effective fence.
In order for a compound to be aromatic, all
FOUR of these criteria
must be met.
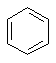
The most important and well
known aromatic system is benzene:
|
Benzene
|
|
|
|
To aid in counting the electrons the
following factors may help:
- Each atom in the
conjugated, cyclic system can only contribute a
maximum
of one pair of electrons (or one orbital)
- "Refining" your
"predicted" hybridisation will allow lone pairs to be
part
of the π system.
- Carbocations and boron
atoms (C+ & B) are sp2 hybridised and have empty "p" orbitals. This allows them to be
part
of the conjugated system but they contribute NO electrons.