| Chapter 20: Carboxylic Acid Derivatives. Nucleophilic Acyl Substitution |
| Chapter 20: Carboxylic Acid Derivatives. Nucleophilic Acyl Substitution |
Reactions of RLi or RMgX with Nitriles
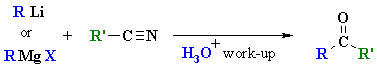
Reaction type: Nucleophilic Acyl Substitution then Nucleophilic Addition
Summary:
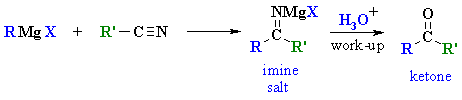
| MECHANISM
FOR THE REACTION OF RMgX WITH A NITRILE |
|
| Step 1: The nucleophilic C in the organometallic reagent adds to theelectrophilic C in the polar nitrile group. Electrons from the C≡N move to the electronegative N creating an intermediate imine salt complex. |
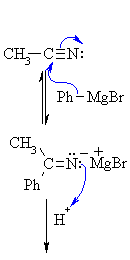  |
| Step 2: An acid/base reaction. On addition of aqueous acid, the intermediate salt protonates giving the imine. |
|
| Step 3: An acid/base reaction. Imines undergo nucleophilic addition, but require activation by protonation (i.e. acid catalysis). |
|
| Step 4: Now the nucleophilic O of a water molecule attacks the electrophilicCwith the π bond breaking to neutralise the change on the N. |
|
| Step 5: An acid/base reaction. Deprotonate the O from the water molecule to neutralise the positive charge. |
|
| Step 6: An acid/base reaction. Before the N system leaves, it needs to be made into a better leaving group by protonation. |
|
| Step 7: Use the electrons on the O in order to push out the N leaving group, a neutral molecule of ammonia. |
|
| Step 8: An acid/base reaction. Deprotonation reveals the carbonyl group ofthe ketone product. |
|
| © Dr. Ian Hunt, Department of Chemistry |