| Chapter 17: Aldehydes and Ketones. Nucleophilic Addition to C=O |
| Chapter 17: Aldehydes and Ketones. Nucleophilic Addition to C=O |
The Baeyer-Villiger Reaction
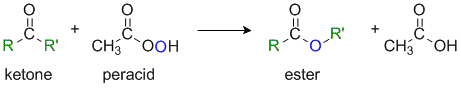
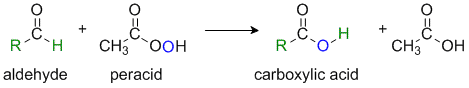
Reaction type: Oxidation-reduction via Nucleophilic addition
Summary
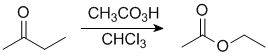 |
In this example, the ethyl group (primary C) migrates in preference to the methyl group, so we end up with an ethyl ester. |
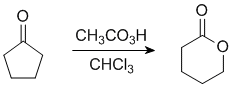 |
| lactone |
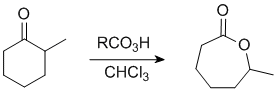
In this example the "O" inserts on the more substituted side.
Study Tip: Count C atoms! |
Related Reactions
THE
BAEYER-VILLIGER REACTION |
|
| Step 1: An acid/base reaction. Protonation of the carbonyl activates it while creating a more reactive nucleophile, the percarboxylate. |
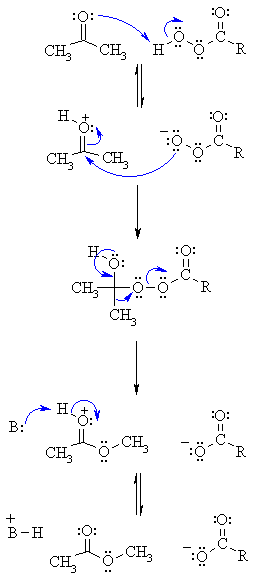 |
| Step 2: Now the nucleophilic O attacks the carbonyl C with the electrons from the C=O π bond going to the positive O. |
|
| Step 3: Electrons from the O come back (this reforms the π bond of the C=O) and we migrate the C-C electrons to form a new C-O bond displacing the carboxylate as a leaving group. |
|
| Step 4: Finally an acid/base reaction reveals the C=O and therefore the ester product. |
|
| © Dr. Ian Hunt, Department of Chemistry |