|
|
|
|
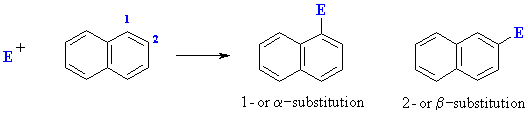
Electrophilic Aromatic Substitution of Polycyclic Aromatics
For example, the nitration of naphthalene proceeds to give mainly 1-nitronaphthalene:
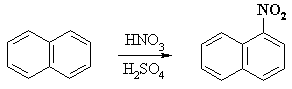
This selectivity can be rationalised by drawing the resonance structures for intermediates produced by attack of the electrophile at the 1- and 2-positions. Attack at the 1- position gives an intermedaite that is represented by 7 resonance contributors of which 4 leave the aromaticity of the other ring intact. In contrast attack at the 2-position gives an intermediate with 6 resonance contributors in which only 2 have the aromaticity of the other ring intact.
| © Dr. Ian Hunt, Department of Chemistry |