| Chapter 10: Conjugation in Alkadienes and Allylic Systems |
| Chapter 10: Conjugation in Alkadienes and Allylic Systems |
Molecular Orbital Analysis of Diels-Alder reaction
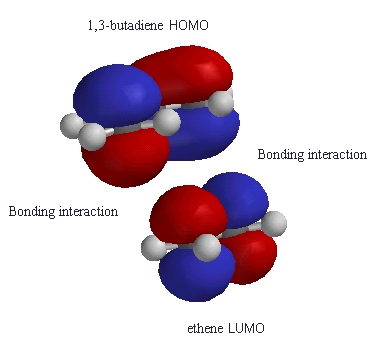
| Notice how the phases of the orbitals at C1 and C4
of the HOMO of butadiene match the phases of the orbitals of C1
and C2 in the LUMO of ethene (i.e. the colours match).
This means there is a favourable bonding interaction at each of these positions, which corresponds to the positions where the two new s bonds are formed. Therefore the reaction is said to be a "symmetry allowed" - this is good news, since we know the reaction works ! |
 |
A more complete analysis using molecular orbital methods (beyond our scope) can also explain:
| © Dr. Ian Hunt, Department of Chemistry |